Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: BEL-treated patients (pts) in clinical trials and long-term extension (LTE) studies1-7 experienced comparable incidence of infections versus those treated with standard therapy (ST) alone in clinical trials1-4; however, sample sizes were limited. We performed the most comprehensive analysis of infections among BEL-treated pts.
Methods: This post hoc analysis pooled data from three multicenter BEL LTE studies of adults with active SLE: LBSL02 LTE (Phase 2; GSK Study 112626),5 BLISS-76 LTE (including US pts only; Phase 3; GSK Study 112233),6 and BLISS-52 + BLISS-76 LTE (excluding US pts from BLISS-76; Phase 3; GSK Study 112234).7 Eligibility for LTE studies required completion of treatment through Week 72 (LBSL02 and BLISS-76 trials), or Week 48 (BLISS-52 trial); improvement in physician global assessment at Week 72 or 68 versus at first BEL dose was also required in LBSL02 LTE. Pts received open-label BEL 10 mg/kg intravenously every 28 days plus ST at the start of each LTE for up to 8 years (BLISS-76 and BLISS-52 + BLISS-76 LTEs) or 13 years (LBSL02 LTE), regardless of prior study drug allocation. Adverse events (AEs) were assessed at each infusion visit until the follow-up visit (post–final BEL dose) and summarized any time post–first BEL dose (in prior trial period or LTE) and in each year.
Results: Of 1304 pts enrolled, 1299 (99.6%) received ≥1 dose of BEL plus ST (pooled safety population), five patients did not receive study treatment, and 604 (46.5%) pts completed their respective studies (cumulative BEL-treated patient-years [PY] on study: 7040.1). Most reported reasons for withdrawal were “withdrawal by patient” (18.3%) and “AE” (10.6%).
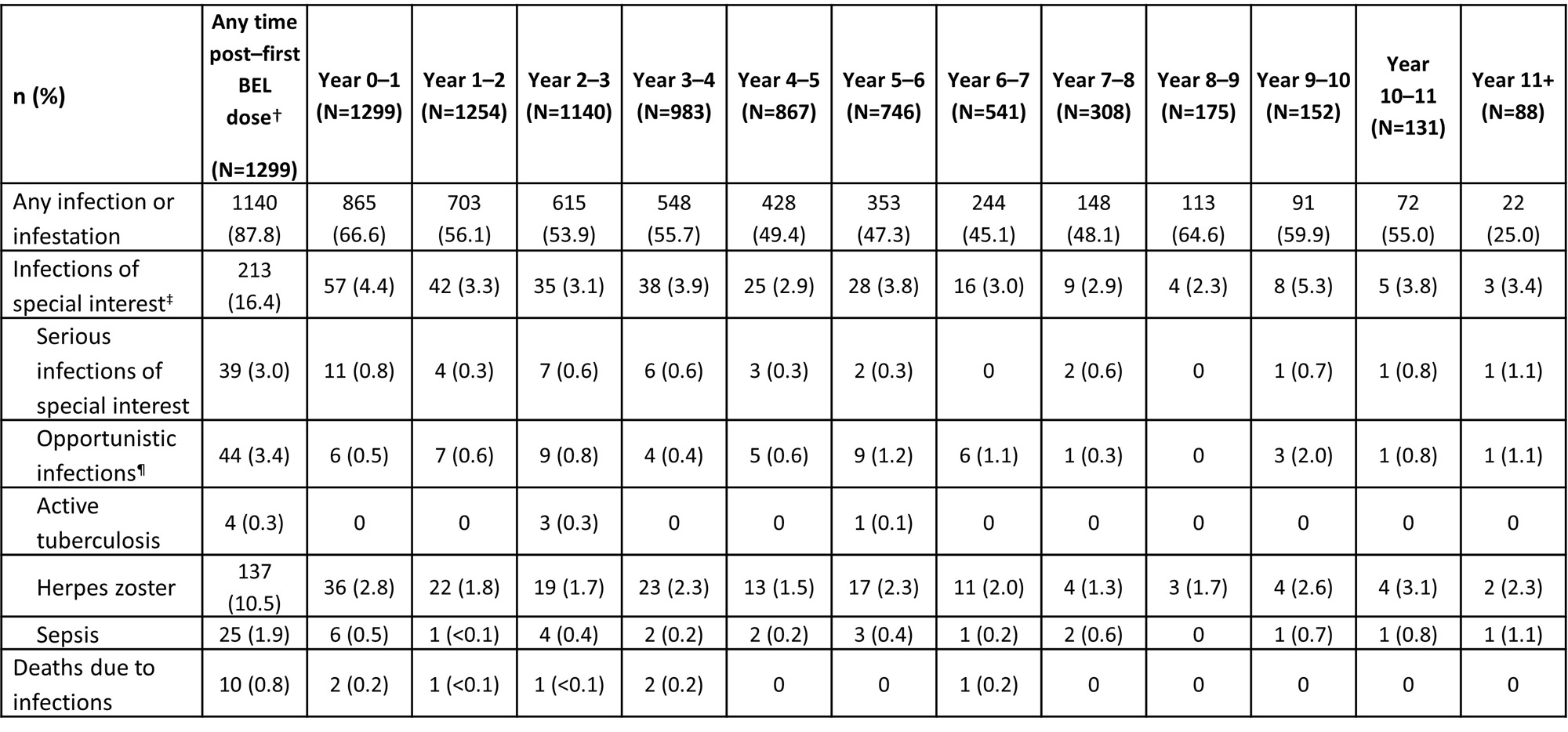
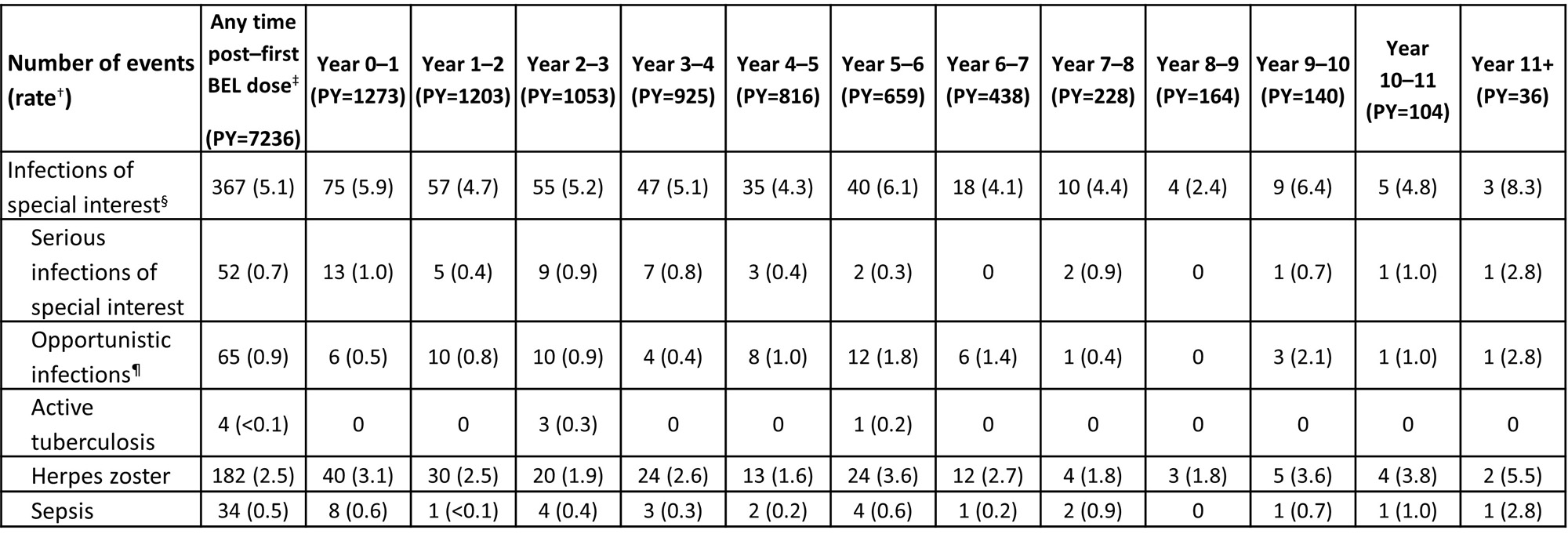
Infections and infestations were the most frequent AE (87.8%) and serious AE (16.9%) at any time post–first BEL dose; 1.9% of discontinuations due to AE were due to infections and infestations. The proportion of pts experiencing an infection of special interest was 16.4% any time post–first BEL dose and generally remained stable each year (Table 1).Herpes zoster was the most frequent infection of special interest; all subcategories generally remained stable over time. The rate of infections of special interest was 5.1 events per 100 PY; all subcategories remained stable over time(Table 2).
Conclusion: In this large, pooled population of BEL-treated pts, incidence of infections was generally stable up to 11 years with no new infection types or increase in severity. Limitations include the open-label nature of the studies, lack of control arm, inclusion of pts who had not withdrawn from prior study periods, and low numbers of pts at later years. Infection incidence rates were similar to or lower than in the GLADEL cohort study8, and among pts receiving ST in the double-blind period of BEL clinical trials.1-4
Funding: GSK
References
1 Sheikh SZ et al. Lancet Rheumatol 2021;3:e122–30
2 Wallace DJ et al. Arthritis Rheum 2009;61:1168–78
3 Furie RA et al. Arthritis Rheum 2011;12:3918–30
4 Navarra SV et al. Lancet 2011;2:721–31
5 Wallace DJ et el. Arthritis Rheumatol 2019;7:1125–34
6 Furie RA et al. Arthritis Rheumatol 2018;6:868–77
7 van Vollenhoven RF et al. Rheumatol (Oxford) 2020;2:281–91
8 Pimentel-Quiroz VR et al. Lupus 2019;28:1101–10
*Infections occurred on or after first BEL dose (in prior trial period or LTE), summarized at any time post–first BEL dose and in each year (post hoc analysis); †post–first BEL dose data include follow-up visits (post–final BEL dose); data from Year 0 up to last visit in the treatment period are shown by years of study participation; pts could be counted in more than one year interval; ‡ infections of special interest are limited to opportunistic infections, active tuberculosis, herpes zoster, and sepsis; ¶per sponsor adjudication.
*Infections of special interest occurred on or after first BEL dose (in prior trial period or LTE), summarized at any time post–first BEL dose and in each year (post hoc analysis); †the rate is the number of events per 100 PY; ‡post–first BEL dose data include follow-up visits (post–final BEL dose); data from Year 0 up to last visit in the treatment period are shown by years of study participation; pts could be counted in more than one year interval; §infections of special interest are limited to opportunistic infections, active tuberculosis, herpes zoster, and sepsis; ¶per sponsor adjudication.
To cite this abstract in AMA style:
Yazdany J, Oku K, Seguro L, Curtis P, Inagaki Y, Levy R, Doria A. Incidence of Infections Among Adult Patients with SLE Treated with Belimumab (BEL): Pooled Data from Three Open-Label Extension Studies over 11+ Years [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/incidence-of-infections-among-adult-patients-with-sle-treated-with-belimumab-bel-pooled-data-from-three-open-label-extension-studies-over-11-years/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-of-infections-among-adult-patients-with-sle-treated-with-belimumab-bel-pooled-data-from-three-open-label-extension-studies-over-11-years/