Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Antimalarials (AM) are frequently used as first-line therapy in mild inflammatory diseases, because of a good benefit/risk ratio. Their most severe side effect is retinopathy, which can potentially lead to blindness, but remains reversible if detected early, provided the treatment is stopped. This complication has been described early [1], but its incidence remains uncertain. A recent update of the American Association of Ophtalmology (AAO) recommendations on screening for chloroquine and hydroxychloroquine retinopathy, suggests to screen patients under AM treatment, with a frequency depending on risk factors, and with the systematic and minimal use of Optical Coherence Tomography (OCT) and Visual Field (VF) test, completed by others tests if required [2]. We aimed at estimating the exact incidence of AM-induced retinopathy, based on available published literature about this issue, with particular reference to detection performed with OCT and VF test.
[1] Hobbs H, Sorsby A, Freedman A, et al. Retinopathy following chloroquine therapy. Lancet 1959.
[2] Marmor MF, Kellner U, Lai TY, et al, American Academy of Ophthalmology Statement. Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision).Ophthalmology 2016.
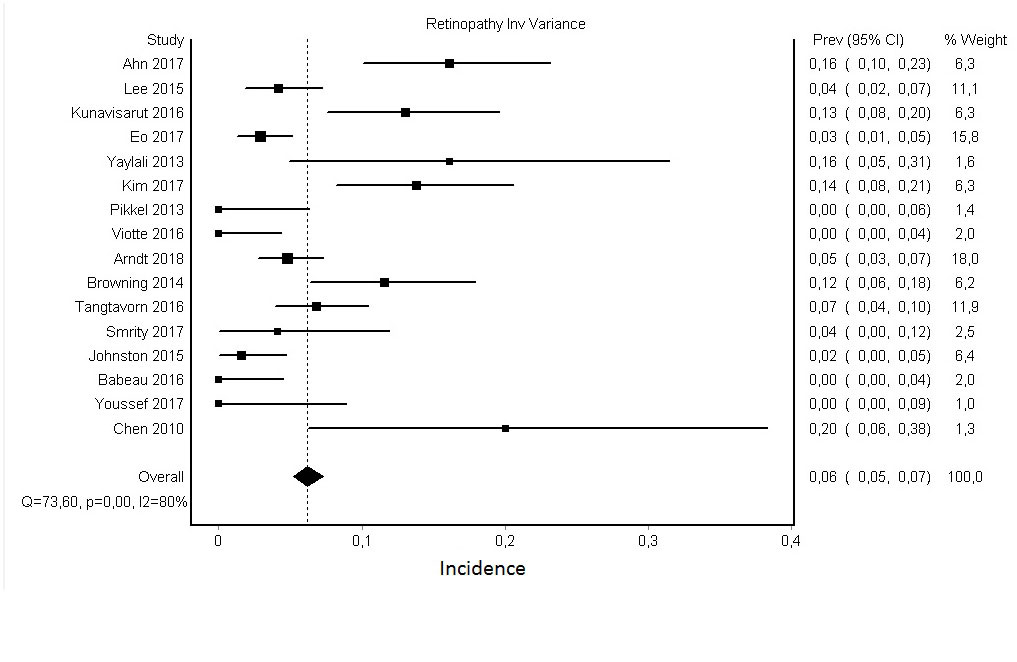
Methods: A systematic literature search was conducted in Pubmed, Cochrane and Embase databases until April 6th 2018, completed by a manual search in references from the resulting selected articles. We first selected all publications about the incidence of retinopathy linked to AM in patients treated for inflammatory diseases and included them in the systematic literature search. Among them, and in order to minimize heterogeneity of results, we focused on those which had used at least OCT and VF test, as recommended by the AAO, to perform a meta-analysis. Analysis was conducted using MetaXL for Microsoft Excel, applying the Inverse of Variance method.
Results: Among the 3890 articles of potential interest, we selected 91 articles appropriately addressing the topic and included them in the systematic literature search. They were dated between 1964 and 2018, with variable population sizes (10 to 3580 patients). Patients were treated with hydroxychloroquine, chloroquine or both for an inflammatory disease (usually lupus or rheumatoid arthritis). Mean treatment duration ranged from 1 to 14,1 years. Most of them were retrospectively designed, and diagnostic method were variate. For the aforementioned meta-analysis, we used data from 16 articles published between 2010 and 2018, in which every patient had at least OCT and VF Test. We found a pooled estimate of prevalence of 6.05% (IC 95% [5.18 – 7.31]), with a heterogeneity coefficient of 80 %.
Conclusion: We found a pooled prevalence of approximately 6% of retinopathy linked to AM when OCT and VF test are used. However diagnostic criteria are not consensually well-defined, leading to heterogeneous data.
To cite this abstract in AMA style:
de Cagny H, Morel J, Gaujoux-Viala C, Combe B, Lukas C. Incidence of Antimalarials-Induced Retinopathy in Inflammatory Rheumatic Diseases, Using OCT and Visual Field Test : A Systematic Review and Meta-analysis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/incidence-of-antimalarials-induced-retinopathy-in-inflammatory-rheumatic-diseases-using-oct-and-visual-field-test-a-systematic-review-and-meta-analysis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-of-antimalarials-induced-retinopathy-in-inflammatory-rheumatic-diseases-using-oct-and-visual-field-test-a-systematic-review-and-meta-analysis/