Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Secondary failure to biologic therapy is challenging and contributes to the complexity of managing psoriatic arthritis (PsA). In this study, we aimed to define the incidence of secondary failure to biologic therapy in patients with PsA and identify the factors associated with its occurrence.
Methods: We retrieved data on patients with PsA followed at our prospective observational cohort who commenced and remained on biologic therapy for at least 1 year. We assessed response at the 1-year point as achievement of ≥40% reduction in the swollen joint count (SJC) and ≥50% reduction in the PASI, or PASI ≤2. We defined secondary failure as the clinician’s judgment of loss of efficacy over time or failure to maintain the response criteria. Patients with secondary failure were compared to those with maintained response in terms of demographic and disease-related characteristics. For factors associated with the development of secondary failure, we used univariate and multivariate Cox regression analyses, adjusting for calendar year (8-year intervals from 2002 to 2024).
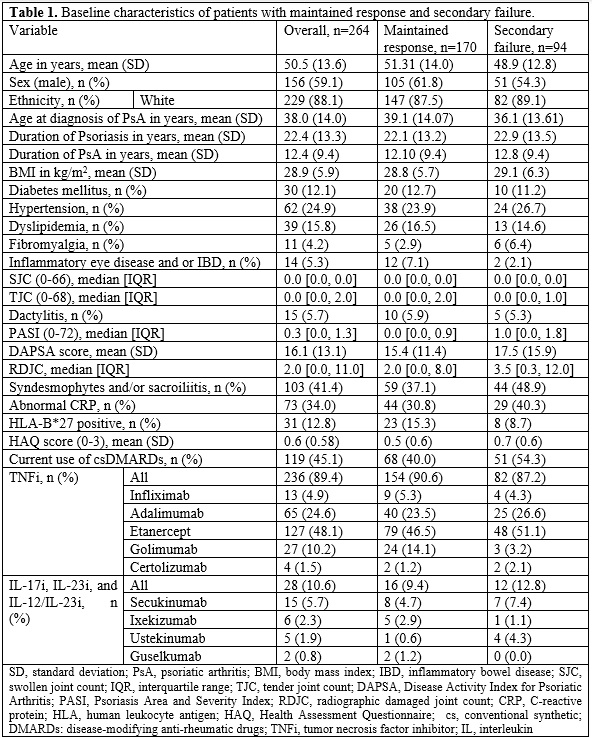
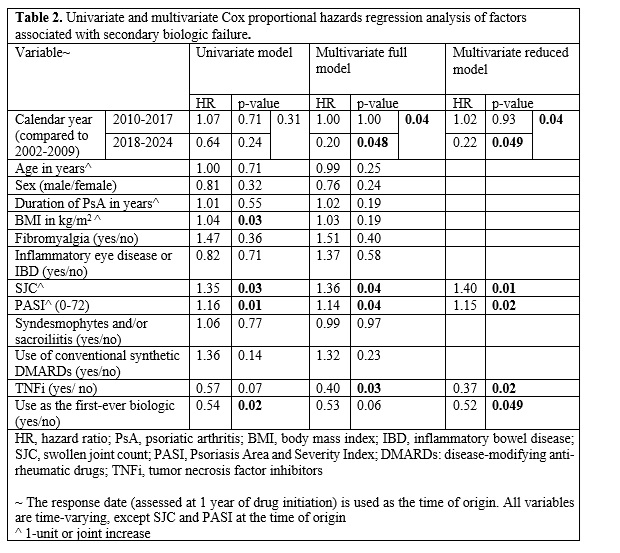
Results: Of the 482 patients who commenced treatment with biologics after clinic enrollment, 264 (54.8%) were classified as responders to therapy at one year. 236 (89.4%) responders received tumor necrosis factor inhibitors (TNFi) (Table 1). 94 (35.6%) responders developed secondary biologic failure at a median [IQR] of 2.7 [1.7, 4.8] years. The incidence rate of secondary failure was 5.96 per 100 person-years. Golimumab had the lowest 5-year prevalence of secondary failure (11.1%). In the reduced multivariate model (Table 2), higher SJC (HR 1.40, p=0.01) and PASI (HR 1.15, p=0.02) at the time of response (1-year point) were associated with the development of secondary failure. Use of TNFi (HR 0.37, p=0.02) and initiation as the first-ever biologic (HR 0.52, p=0.049) were associated with a lower incidence of secondary failure.
Conclusion: Secondary biologic failure is common in PsA. A more complete clinical response, use of TNFi, and commencement as the first-ever biologic are all associated with persistence of therapy.
To cite this abstract in AMA style:
Kharouf F, Gao S, Alhadri A, Pereira D, Cook R, Chandran V, Gladman D. Incidence and Predictors of Secondary Failure to Biologic Therapy in Patients with Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/incidence-and-predictors-of-secondary-failure-to-biologic-therapy-in-patients-with-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-and-predictors-of-secondary-failure-to-biologic-therapy-in-patients-with-psoriatic-arthritis/