Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The antimalarial drugs are considered safe and well tolerated, with low risk of side effects. Hydroxychloroquine is considered safer but less effective than chloroquine, although the choice remains a matter of discussion and it generally depends on the experience. Both can cause ocular toxicity by corneal and retinal deposition which produces irreversible visual disturbances and the patient may not perceive its presence at an early stage. The American Academy of Ophthalmology recommendations for screening of chloroquine (CQ) and hydroxychloroquine (HCQ) retinopathy recommend to use more sensitive test than fundus exam such as Spectral Domain Optical Coherence Tomography (SD-OCT) to detect early retinal toxicity. The objective was to study the incidence of retinal toxicity in patients treated with
antimalarials in a Rheumatology Unit and to evaluate the effectiveness of SD-OCT in the screening of hydroxichloroquine and chloroquine toxicity
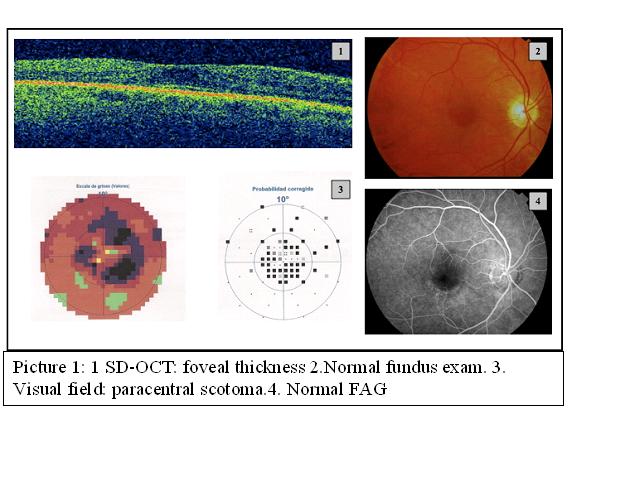
Methods: Descriptive study evaluating 39 patients treated with antimalarials who were referred to ophthalmology to study retinal toxicity during 2011. Data collection included demographic data of patients, type of antimalarial prescribed, daily and cumulative doses, rheumatic disease, corticosteroid use, associated morbidity, visual acuity, examination of the anterior pole, fundus exam, visual field, SD-OCT and, in case that it shows any sign of toxicity, multifocal electroretinogram. Fluorescein angiography (FAG) was made to patients that showed macular disturbances.
Results: Three patients were treated with CQ and 36 with HCQ. Five of 39 patients had alterations in the SD-OCT, two of them had typical fundus appearance and three didn’t show significant changes in visual acuity or retinal imaging. Visual field and electroretinogram helped to confirm early toxicity. Among the patients with retinopathy, three (100%) had been treated with CQ and two (5.5%) with HCQ. The average duration of treatment in these patients was 2.37 + / -1.37 years and the mean cumulative dose of CQ was 559 g and 172.5 g for HCQ. There was no statistically significant association between retinal toxicity and sex, age, rheumatic disease, duration or dose of treatment with p value greater than 0.05.
Conclusion: The incidence of retinal toxicity in our patients treated with antimalarial drugs was 12.8%. Despite the small sample size to prove statistical significance, CQ was the antimalarial that caused most of cases of retinopathy and the SD-OCT was the most sensitive test to detect it. It is essential a fluent communication between Rheumatology and Ophthalmology to have a proper follow-up of the patients treated with CQ and HCQ and an early detection of retinopathy.
Disclosure:
S. Soro Marin,
None;
A. D. C. Haro Martínez,
None;
D. Palma Sanchez,
None;
M. D. R. Gonzalez Molina,
None;
M. Mayor Gonzalez,
None;
E. Rubio Velazquez,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/incidence-and-early-detection-of-retinal-toxicity-in-patients-treated-with-chloroquine-and-hydroxichloroquine-in-rheumatology-practice/