Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Management of Systemic lupus erythematosus (SLE) requires accurate, timely disease activity monitoring for optimal treatment and research. The SLEDAI-2K provides objective clinical and laboratory data, but inconsistent completion rates limit its utility. This study aimed to determine baseline SLEDAI-2K documentation rates in our pediatric rheumatology clinic and implement interventions to improve them using quality improvement (QI) methodology.
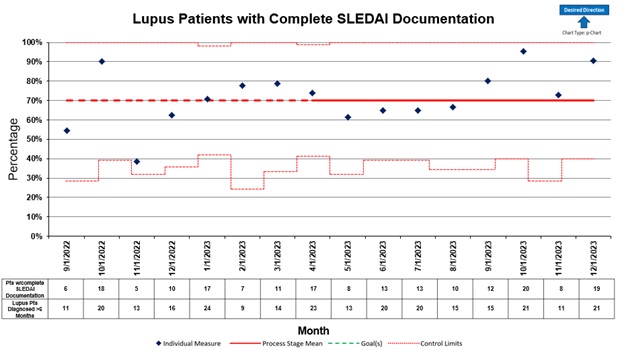
Methods: A QI team reviewed all SLE clinical encounters with a physician over an eight-month period to establish baseline documentation rates. We defined a 30-day window before and after the clinic visit for SLEDAI-2K clinical and laboratory data collection. SLEDAI completion rates were tracked monthly using a p-chart. Pareto charts identified areas for improvement. Results were shared with providers during monthly QI meetings.
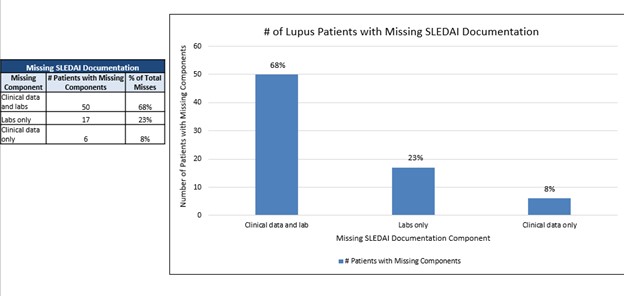
Results: We identified incomplete provider-entered clinical data and delayed laboratory results as major barriers to SLEDAI-2K completion. Our first Plan-Do-Study-Act (PDSA) cycle involved implementing a “soft-stop” in the electronic health record (EHR) to alert providers of missing SLEDAI-2K documentation at time of chart closure. This alert was updated from a previous QI initiative. Additionally, we introduced an “ordered and pending” option with electronic data retrieval to allow for completion the same day. Lastly, we implemented an automated laboratory test result report outside of the EHR to obtain relevant results for SLEDAI completion, eliminating the need for providers to enter this data manually. A consolidated report caprtured all SLEDAI components for detailed analysis of missing components. Baseline documentation rates were 70%. After implementing the PDSA cycle, rates started to improve. Completion rates were most impacted by the implementation of the EHR softstop and automation of laboratory results
Conclusion: This project demonstrates the effectiveness of leveraging the EHR to improve SLEDAI-2K documentation. Soft-stops and electronic data retrieval addressed identified barriers and facilitated timely completion. Our QI initiative successfully improved SLEDAI-2K documentation, enhancing data quality for clinical care and research.
To cite this abstract in AMA style:
Stojkic I, Hardison V, Barbar-Smiley F, Kallash M, Patel H, Hughes J, Gallop J, Leone A, Duff E, Taxter A, Ardoin S, Sivaraman V. Improving SLEDAI-2K Documentation for Childhood-Onset Systemic Lupus Erythematosus (cSLE) in a Pediatric Rheumatology Clinic Using Quality Improvement Methodology [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/improving-sledai-2k-documentation-for-childhood-onset-systemic-lupus-erythematosus-csle-in-a-pediatric-rheumatology-clinic-using-quality-improvement-methodology/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-sledai-2k-documentation-for-childhood-onset-systemic-lupus-erythematosus-csle-in-a-pediatric-rheumatology-clinic-using-quality-improvement-methodology/