Session Information
Date: Sunday, November 10, 2019
Title: Measures Of Healthcare Quality Poster I: Testing, Screening, & Treating
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Hydroxychloroquine (HCQ) prescribing is standard of care for patients with SLE. The main potential side effect is retinal toxicity, especially at higher doses and with prolonged use. In 2016, the American Academy of Ophthalmology (AAO) modified its HCQ dosing and screening guidelines to recommend a maximum daily dose of 5 mg/kg of actual body weight and retinal examinations at baseline, then annually after 5 years of taking HCQ. We undertook a quality improvement project in a safety net rheumatology clinic that had two aims: 1) to increase the proportion of patients prescribed hydroxychloroquine at recommended doses from 85% at baseline to 95% and 2) to improve the proportion of patients receiving appropriate retinal screening from 52% at baseline to 80% at one year.
Methods: Using the Institute for Healthcare Improvement’s Model for Improvement, we first mapped the existing clinical workflow for HCQ prescribing and monitoring. We developed and applied two process measures to track our progress: the proportion of patients prescribed hydroxychloroquine receiving a dose less than or equal to 5 mg/kg at their last encounter and the proportion of patients prescribed hydroxychloroquine at their last encounter with a retinal screening test over the last 18 months. After identifying baseline values for our two process measures, we worked with clinicians and clinic support staff over four Plan-Do-Study-Act (PDSA) cycles to develop our intervention. The final optimized workflow involved the clinic’s nurse using a weekly report to identify patients who were overdue for retinal screening for an ophthalmology referral and to alert physicians whose patients were on a higher than recommended dose of HCQ. In September 2018, we queried the electronic health record again for data on our clinic’s adherence rates to AAO guidelines between September 2017 to August 2018 to analyze the impact of our intervention.
Results: The percentage of clinic patients prescribed HCQ at doses less than 5.0 mg/kg daily increased from 84% to 86% during the study period, and the percentage receiving retinal screening increased from 64% to 76%. Of note, only 8 out of the 243 SLE patients on HCQ with a rheumatology office visit in 2018 did not have an ophthalmology appointment scheduled. The remainder of those that did not have retinal screening had an ophthalmology appointment scheduled, but either cancelled or did not appear to their appointments.
Conclusion: Using the Model for Improvement, we were able to develop and integrate an intervention to increase adherence to the AAO recommendations for HCQ dosing and retinal screening measures, although we fell short of our pre-specified improvement goals. Although the intervention has been moderately effective at improving patient safety practices for this drug, efforts are ongoing to further understand barriers to further improvement, including root cause analysis of individual-level and systems-level factors.

Figure 1_ACR 2019_Castillo_Dodge
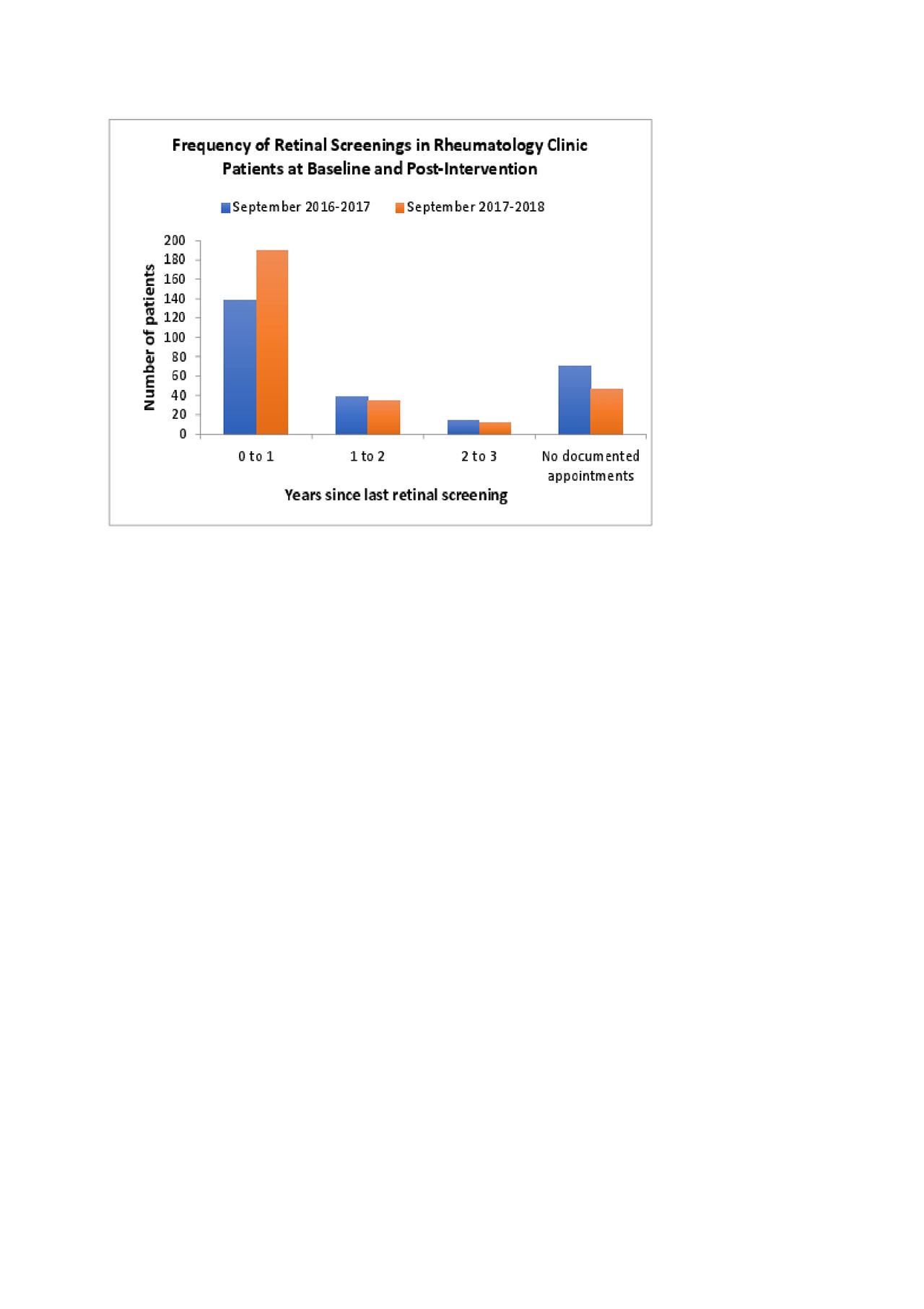
Figure 2_ACR 2019_Castillo_Dodge
To cite this abstract in AMA style:
Castillo F, Dodge M, Noh J, Trupin L, Yazdany J, Goglin S. Improving Safe Prescribing of Hydroxychloroquine in a Safety Net Hospital Rheumatology Clinic [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/improving-safe-prescribing-of-hydroxychloroquine-in-a-safety-net-hospital-rheumatology-clinic/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-safe-prescribing-of-hydroxychloroquine-in-a-safety-net-hospital-rheumatology-clinic/
