Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose:
Streptococcus
pneumoniae is a leading
cause of severe infections, including pneumonia and meningitis, among
immunocompromised patients. The administration of both the 13-valent
pneumococcal conjugate vaccine (PCV13) and the 23-valent pneumococcal
polysaccharide vaccine (PPSV23) is indicated for patients with
immunocompromising conditions per U.S. Advisory Committee on Immunization
Practices (ACIP) guidelines.
The
SMART aim of this project was to increase the percentage of immunocompromised
patients 11 years and older seen in the Rheumatology and Inflammatory Bowel Disease
(IBD) clinics at Cincinnati Children’s Hospital Medical Center (CCHMC) who
receive PCV13 vaccine from 20% to 80%.
Methods:
Providers from various
specialty clinics at CCHMC recognized common barriers related to immunization administration,
including knowledge gaps, access to immunization records, identification of the
immunocompromised patient, sharing responsibility of vaccine administration
between the primary care provider (PCP) and the specialist and the appropriate
timing of vaccines.
Eligible
patients included adolescents defined as immunocompromised according to Center
for Disease Control and Prevention (CDC) guidelines, between 11-18 years of
age, who were seen in the Rheumatology and IBD clinics between February and June
2015.
Interventions
that were implemented during the study included education of clinic providers
and nurses; the development of physician “talking points” for patients; easy
clinic access to vaccines and pre-visit planning that included a visual
reminder to the provider and a pended order for the vaccine.
Results:
During the 19-week
study period, 443 eligible patients were seen in both clinics. The
pre-intervention immunization rate for PCV13 ranged from 0% – 20%. Pending the vaccine
order increased the percentage to ~60% [Figure 1] and the addition of provider “talking
points” increased it further to 80%. In total, 376 patients (84.8%) received
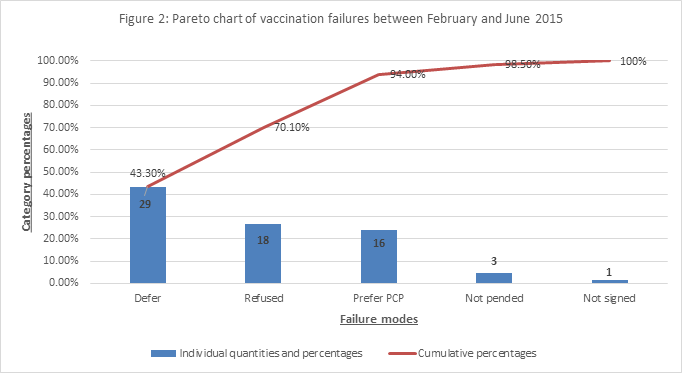
the PCV13. At the initiation of the project, most failures were related to the
preference to receive vaccine in the PCP’s office. With the institution of the
“talking points”, the main reason for failure was a request to defer the
vaccine to a later visit with the specialist (Figure 2).
Conclusion:
We have demonstrated
that with a few key interventions, it is possible to increase the PCV13 vaccination
rate to over 80% in a heterogeneous group of immunocompromised patients. These
same measures can be easily implemented in other clinics for different
vaccinations.
To cite this abstract in AMA style:
Feldon M, Furnier A, Lehmann C, Fletcher B, Kues J, Speer B, Kramer S, Morgan P, Siegle L, Brady R, Huggins JL. Improving Pneumococcal Immunization Rates Among Immunocompromised Adolescent Patients at a Tertiary Care Children’s Hospital [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/improving-pneumococcal-immunization-rates-among-immunocompromised-adolescent-patients-at-a-tertiary-care-childrens-hospital/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-pneumococcal-immunization-rates-among-immunocompromised-adolescent-patients-at-a-tertiary-care-childrens-hospital/