Session Information
Date: Saturday, November 12, 2022
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Hydroxychloroquine (HCQ) is a medication used in most patients with systematic lupus erythematous (SLE). It is generally a safe; however, it can cause retinal toxicity and lead to blindness. A study published in JAMA in 2014 reported a prevalence of 7.5% of HCQ related eye disease (Melles and Marmor, 2014). Screening guidelines were then updated in 2016 by the American Academy of Ophthalmology (AAO) (Marmor et al, 2016) recommending baseline screening within in a year of starting the medication and annually after five years of continued use. The purpose of this quality improvement project was to assess how well screening guidelines were followed and if an educational intervention improved compliance to these guidelines.
Methods: Patients > 18 with SLE who are actively using HCQ were evaluated. Data collection included demographics, if the patient is on a < 5mg/kg dose, physician documentation on eye screening status, eye screening status, & available documentation by an eye specialist in the chart. An educational intervention using a lecture format was done with the physicians reviewing screening guidelines, compliance with meeting them, and the tool (packet) being implemented to improve compliance. The packet handouts focused on improving consent & physician documentation. A repeat chart review was performed collecting the same data. Changes in compliance from pre- to post-intervention was assessed using the chi-squared test with p value less than 0.05 to be significant.
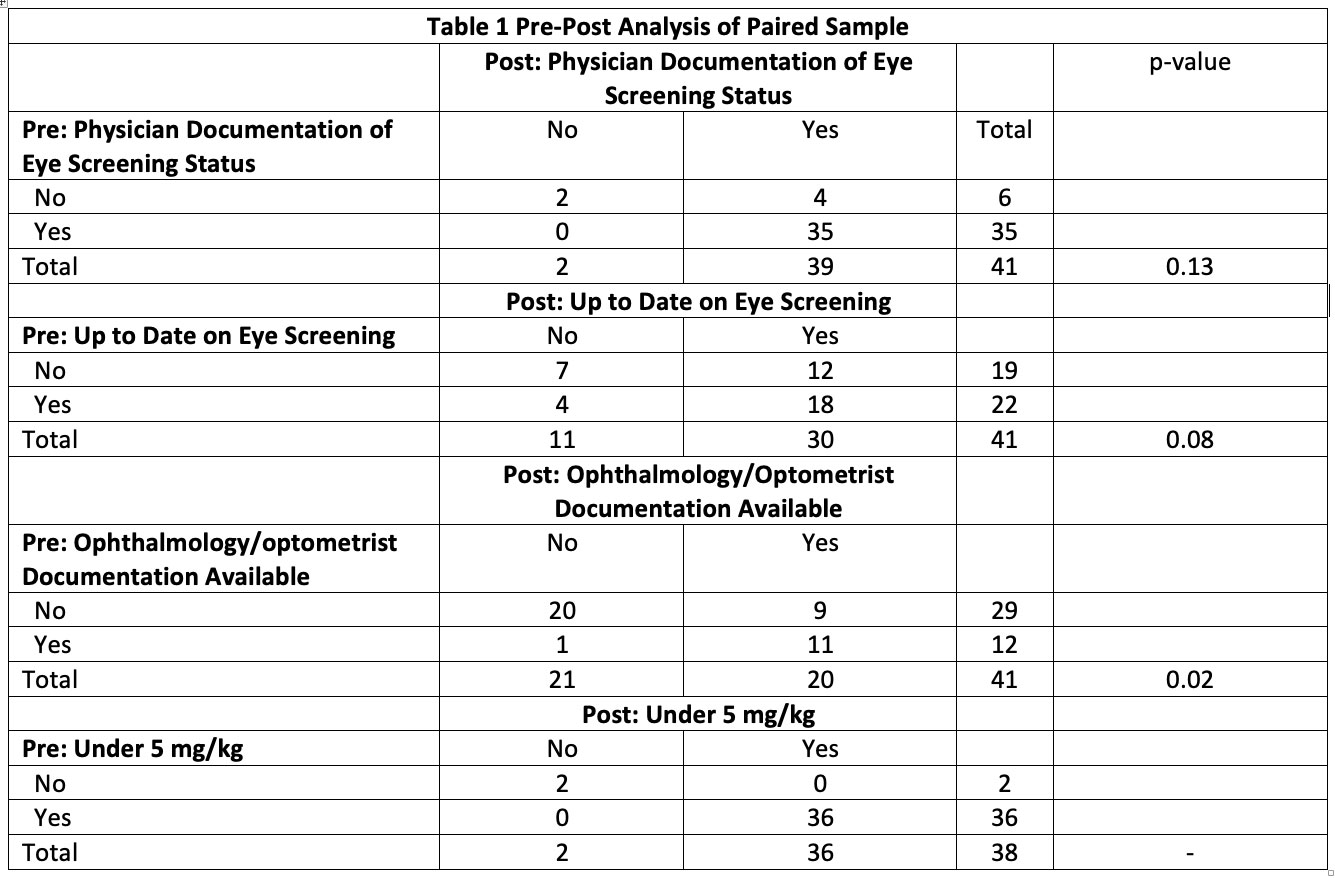
Results: After the intervention there was a general improvement in physician documentation and screening rates seen. For directly paired analysis shown in table 1, screening rate improved from 53.7% (22/41) to 73.2% (30/41) approaching statistical significance p = 0.08. Available eye specialist documentation improved 29.2% (12/41) to 48.8% (20/41) that was statistically significant p = 0.02.
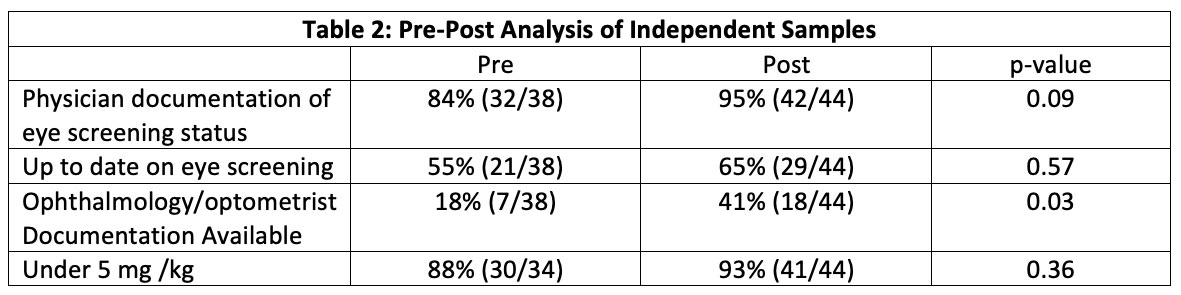
In the analysis of independent samples seen in table 2, physician documentation improved from 84.2% (32/38) to 95.5% (42/44) approaching statistical significance p = 0.09. Available eye specialist documentation improved from 18% (7/31) to 41% (26/44) with statistical significance of p = 0.03.
There was no significant change in patients under 5 mg/kg dosing of HCQ in either analysis groups although this was a high percentage on initial analysis.
Conclusion: Screening for hydroxychloroquine eye toxicity is still very important and initial screening revealed that it is a major need for improvement in a community hospital setting. There are likely several barriers to getting timely screening including lack of in-house ophthalmologist, difficulties with insurance, awareness or understanding the seriousness of HCQ eye toxicity, poor documentation, and even access to specialists due to COVID. This study showed that an educational lecture with physicians and an easy-to-use tool focused on minimizing these barriers improved eye screening for HCQ ocular toxicity.
To cite this abstract in AMA style:
Gennaoui G, Kang S, Dhar J. Improving Eye Screening Compliance in Patients Taking Hydroxychloroquine in a Community Hospital Setting [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/improving-eye-screening-compliance-in-patients-taking-hydroxychloroquine-in-a-community-hospital-setting/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-eye-screening-compliance-in-patients-taking-hydroxychloroquine-in-a-community-hospital-setting/