Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Prior studies of RISE Registry found 9-11% of women of reproductive age with contraception documentation. We sought to identify and assess methods to increase contraception documentation within RISE Registry sites.
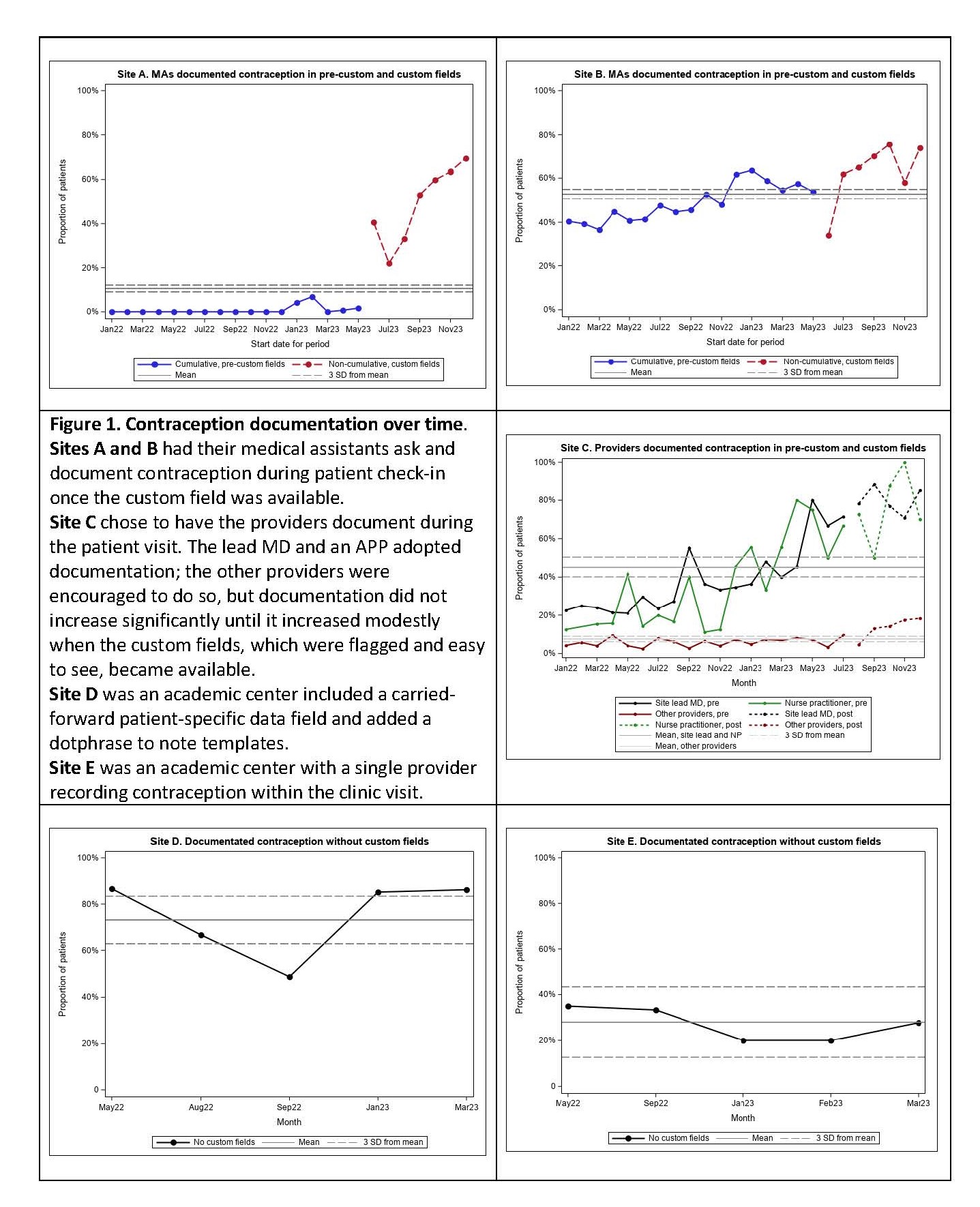
Methods: We used a Learning Collaborative (LC) model to bring together experts and clinical rheumatologists from RISE Registry clinics every 4-8 weeks for 2 years. The LC determined a quality measure and data they considered both clinically important and feasible to collect, as well as data collection and reporting methods. Data from community clinics (Sites A, B, C) was extracted via EMR reporting of individual patients; academic centers (Sites D, E) reported aggregate data for up to 30 patients per month. Data are presented in run charts with the overall mean and 3 standard deviations (SD). Consecutive rates more than 3 SD from the mean indicate a statistically significant pattern change.
Results: Providers from 7 clinics joined the LC and 5 clinics actively participated and provided data. The LC defined the quality metric: Among individuals of reproductive potential ages 15 to 44, the percentage that had documentation of contraceptive use, non-use, or infecundity. The LC also elected to document interest in pregnancy and to complete these data for all females of reproductive age at every visit.
Documentation location: All sites originally had a carried-forward patient-specific data-field for contraception and most did not use this field. None had a pregnancy intention data field. The LC determined that flagged, easy-to-access, visit-specific data-fields would facilitate data collection; these were created within the community practice EMRs. Academic sites put a dotphrase into note templates.
Method of documentation: Sites A and B elected to have medical assistants ask and document the data during check-in. Site C, D, and E elected to have the providers ask and document during the encounter.
Improving documentation: Once the custom fields were in place, Sites A & B had rapid uptake of contraception documentation (Figure 1) and pregnancy interest with significant improvement over the next 5 months. At Site C, 2 providers had significant improvements over time; the remaining providers only had a modest increase with the custom field. Site D had high use of the contraception dotphrase throughout. Site E had moderate levels of documentation that did not increase.
Patient responses: Among the 1057 women with documentation of pregnancy intention, 7.6% reported that ‘yes,’ they were interested in pregnancy in the next year, while 2.7% were either ‘unsure’ or ‘OK either way.’ Within a six-month period, of the 109 who reported yes, unsure, or OK either way, 13% had a change in their response.
Conclusion: Routinely documenting contraception and pregnancy interest is feasible in academic and community rheumatology practices. Documentation is enhanced by well-placed, flagged, visit-specific data fields and completion by medical assistants or very engaged providers. An estimated 10% of women with rheumatic disease of reproductive age are interested in pregnancy, a state that can change over the course of 6 months.
To cite this abstract in AMA style:
Clowse M, Bajaj P, Bermas B, Chiesa J, Dao K, Freeman P, Gujar B, Hill B, Jones K, Jones R, Marslett A, Mills B, Roberts J, Snyderman A, Zulig L. Improving Documentation of Contraception and Pregnancy Intention in Rheumatology Practice [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/improving-documentation-of-contraception-and-pregnancy-intention-in-rheumatology-practice/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-documentation-of-contraception-and-pregnancy-intention-in-rheumatology-practice/