Session Information
Date: Sunday, November 10, 2019
Title: Measures Of Healthcare Quality Poster I: Testing, Screening, & Treating
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Mycophenolate is widely prescribed in the rheumatology setting. However, usage of mycophenolate during the first trimester of pregnancy is associated with an increased risk of congenital malformations and miscarriages. The Risk Evaluation and Mitigation Strategy (REMS) from the Food and Drug Administration recommends pregnancy screening at routine follow-up visits. However, there is currently no standardized method of ensuring routine pregnancy screening. After a patient required pregnancy termination due to major fetal abnormalities, this quality improvement project sought to improve physician adherence in pregnancy screening for female patients on mycophenolate.
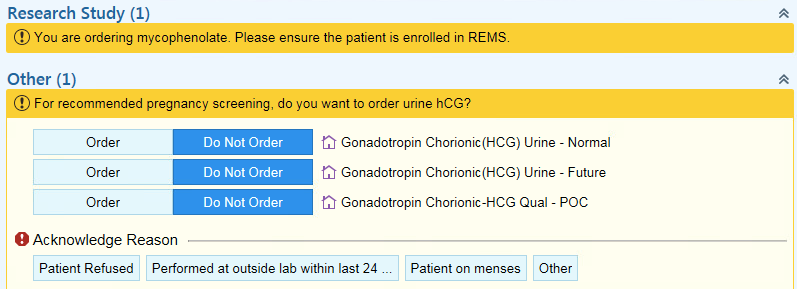
Methods: This project was conducted in the pediatric rheumatology clinic setting at a single tertiary care center. Female patients age 10 and older prescribed mycophenolate mofetil or mycophenolic acid were included in the study. Process mapping was utilized to identify potential areas of improvement. Two Plan-Do-Study-Act (PDSA) cycles were completed. The identified intervention included leveraging the electronic medical record through the development of a Best Practice Advisory (BPA) tool (Figure 1). The BPA alert was designed to prompt ordering of a urine human chorionic gonadotropin (hCG) test for female patients age 10 and older prescribed mycophenolate. The current PDSA cycle included analysis from March 2018 to March 2019, and missed opportunities for pregnancy screening were identified. A missed opportunity was defined as an encounter where mycophenolate was prescribed but a urine hCG was not ordered within 7 days.
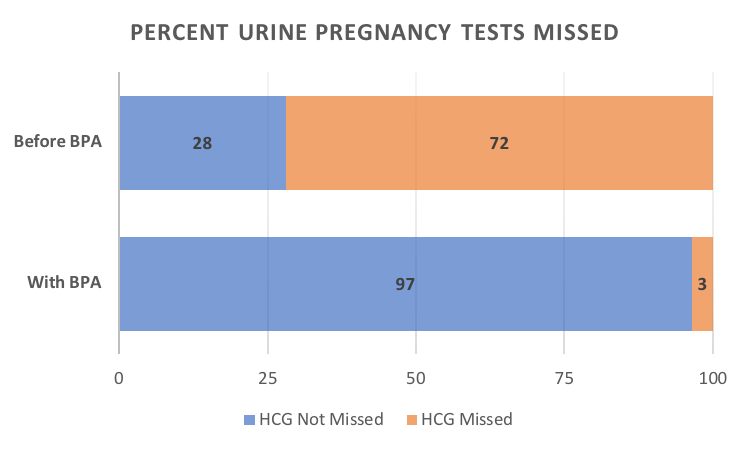
Results: At baseline, pregnancy screening was performed at 11% (8/71) of the encounters where mycophenolate was prescribed. Implementation of the BPA alert resulted in marked improvement in routine pregnancy screening to 79% (114/144, p< 0.00001). An opportunity for routine urine pregnancy screening was missed 72% (51/71) of the time at baseline. Implementation of the BPA alert resulted in reduction of the missed opportunities to 3% (5/144, p< 0.00001), shown in Figure 2. Authorization of a medication refill prior to the next visit was the most common reason for a missed opportunity. Analysis revealed that the BPA alert was not triggered if mycophenolate was prescribed outside a clinic visit.
Conclusion: Pregnancy screening in our female patients of childbearing age on a teratogenic medication is paramount. Effective utilization of an alert system embedded in the electronic medical record resulted in a striking improvement in routine urine pregnancy screening. This systems-based approach provides a sustainable infrastructure for improvement in patient care.
To cite this abstract in AMA style:
Do V, Nguyen M, Akamine K, Fuller J, Nassi L, Wright T, Stewart K. Improving Adherence to Pregnancy Screening in Patients on Teratogenic Medications Using an Electronic Medical Record Alert System: A Quality Improvement Initiative [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/improving-adherence-to-pregnancy-screening-in-patients-on-teratogenic-medications-using-an-electronic-medical-record-alert-system-a-quality-improvement-initiative/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improving-adherence-to-pregnancy-screening-in-patients-on-teratogenic-medications-using-an-electronic-medical-record-alert-system-a-quality-improvement-initiative/