Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with active psoriatic arthritis (PsA) may experience disease manifestations across multiple domains, as well as impaired functioning in daily activities at home and at work. ACTIVE, a phase 3b study, is evaluating the efficacy of apremilast (APR) monotherapy in biologic-naïve subjects with PsA who may have had exposure to 1 prior conventional disease-modifying anti-rheumatic drug. The ACTIVE work productivity findings through Week 104 are reported.
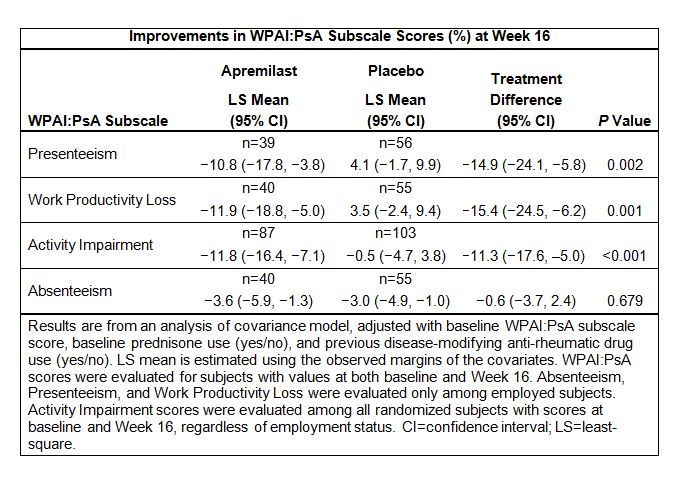
Methods: Subjects were randomized (1:1) to receive APR 30 mg BID or placebo. Subjects who did not improve at least 10% in both swollen and tender joint counts at Week 16 were eligible for early escape. At Week 24, all remaining placebo subjects were switched to APR. Work productivity and activity impairment were assessed at baseline and Week 16 using the 6-item, self-administered Work Productivity and Activity Impairment Questionnaire: Psoriatic Arthritis (WPAI:PsA). The WPAI:PsA includes 4 subscale scores: Absenteeism, Presenteeism, Work Productivity Loss, and Activity Impairment; subscale scores each range from 0% to 100%, with higher scores indicating greater impairment. Work-related subscales were evaluated only among employed subjects, while activity impairment was evaluated among all subjects, regardless of employment. Correlations were examined at Week 16 between WPAI:PsA subscale scores and selected 36-item Short-Form Health Survey version 2 (SF-36v2) domain scores (i.e., Physical Functioning [PF], Bodily Pain [Pain], and Vitality [VIT]). Improvement in work productivity was assessed through Week 104.
Results: Baseline characteristics were similar between the APR and placebo subjects with WPAI:PsA scores included in the current analysis. At Week 16, APR significantly improved work productivity and the ability to carry out daily activities compared with placebo, with significantly greater mean improvements observed in the overall Work Productivity Loss score (P=0.001) and Activity Impairment score (P<0.001) (Table). Estimated mean change in the Absenteeism score was similar with APR vs. placebo (P=0.679). By contrast, the Presenteeism score showed significant improvement with APR vs. worsening with placebo (−10.8% vs. 4.1%; P=0.002). At Week 16, statistically significant correlations were observed between WPAI:PsA subscale scores (except Absenteeism) and SF-36v2 domain scores (i.e., PF, Pain, and VIT). Among subjects randomized to receive APR at baseline, Week 16 WPAI:PsA subscale score improvements were generally maintained through Week 104.
Conclusion: In biologic-naïve subjects with active PsA, APR monotherapy contributed to an overall improvement in work productivity at Week 16, which correlated with SF-36v2 PF, Pain, and VIT scores; improvements in WPAI:PsA subscale scores were generally maintained to Week 104.
To cite this abstract in AMA style:
Mease PJ, Gladman DD, Davenport EK, Zhou X, Guerette B, Teng L, Kaura S, Nash P. Improvements in Work Productivity with up to 104 Weeks of Apremilast Monotherapy: Results from a Phase 3b, Randomized, Controlled Study in Biologic-NaïVe Subjects with Active Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/improvements-in-work-productivity-with-up-to-104-weeks-of-apremilast-monotherapy-results-from-a-phase-3b-randomized-controlled-study-in-biologic-naive-subjects-with-active-psoriatic-arthritis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvements-in-work-productivity-with-up-to-104-weeks-of-apremilast-monotherapy-results-from-a-phase-3b-randomized-controlled-study-in-biologic-naive-subjects-with-active-psoriatic-arthritis/