Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
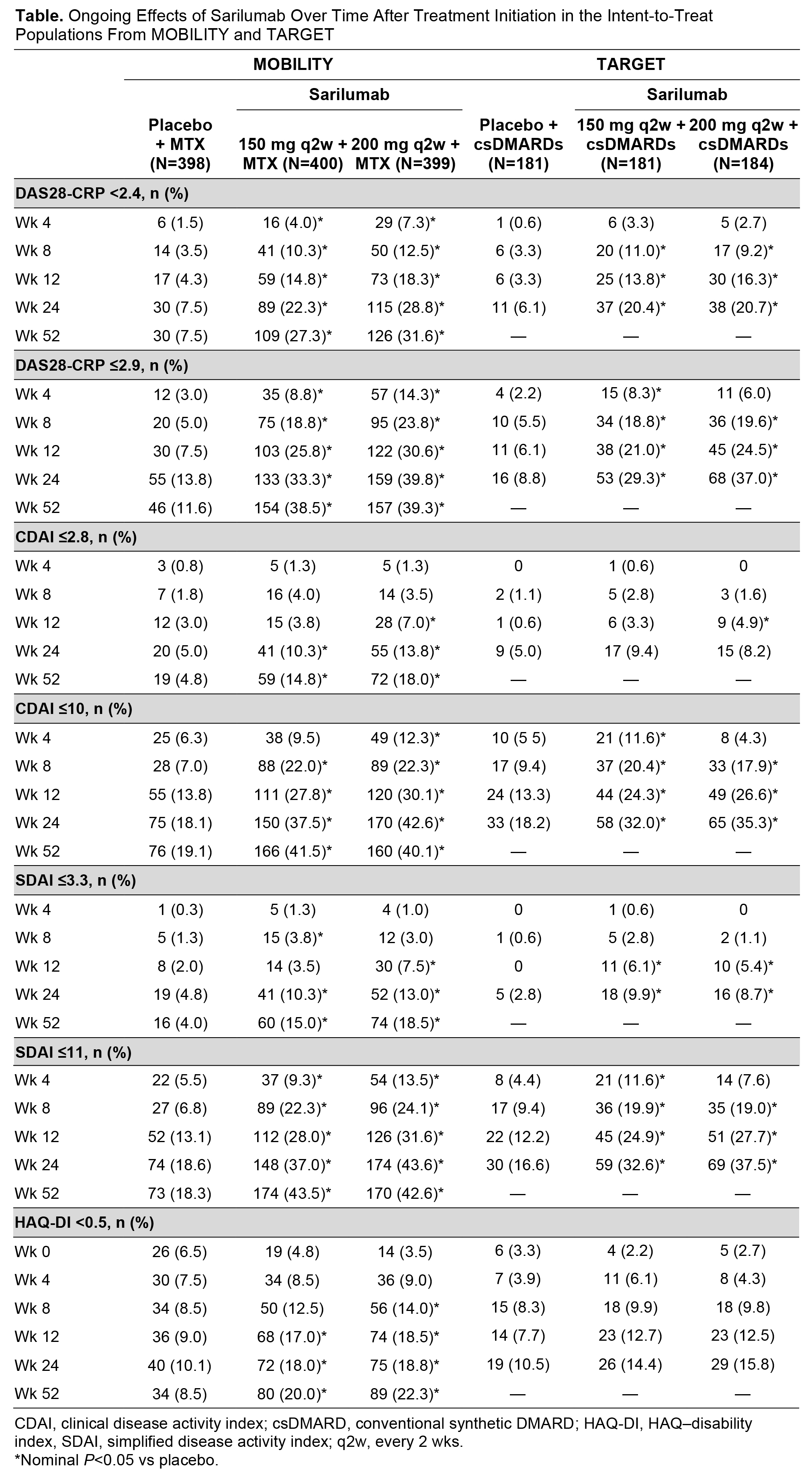
Background/Purpose: Sarilumab is a human mAb blocking the IL-6Rɑ. Efficacy and safety of sarilumab (150 or 200 mg subcutaneously every 2 wks [q2w]) + conventional synthetic (cs)DMARDs were demonstrated in patients with active, moderate-to-severe RA in 2 phase 3 studies: the 52-wk MOBILITY study (NCT01061736) in patients with inadequate response to MTX (MTX-IR) and the 24-wk TARGET study (NCT01709578) in patients with inadequate response to or intolerance of TNF inhibitors (TNF-IR). The most common treatment-emergent adverse events in both studies included infections, neutropenia, injection site reactions, and increased transaminases. This post hoc analysis assessed benefit of sarilumab treatment by examining the proportion of patients who achieved treat-to-target goals of remission or low disease activity (LDA) by 1 year in MOBILITY and 6 months in TARGET.
Methods: Adults with active RA were randomized 1:1:1 to receive sarilumab 150 or 200 mg q2w or placebo + background MTX (MOBILITY) or csDMARDs (TARGET). Clinical efficacy was evaluated for remission and LDA using DAS28-CRP (<2.4 and ≤2.9),1,2 clinical disease activity index (CDAI; ≤2.8 and ≤10), and simplified disease activity index (SDAI; ≤3.3 and ≤11) at wks 4, 8, 12, and 24 in TARGET and additionally at week 52 in MOBILITY. Functional remission (HAQ–Disability Index [HAQ-DI] <0.5)3 was also assessed.
Results: In both studies, more sarilumab-treated patients achieved remission (DAS28-CRP only) and LDA (all criteria) vs placebo (Table). Although remission and LDA generally became evident between wks 4 and 8 in most patient groups (nominal P<0.05 vs placebo, both doses), with ongoing sarilumab treatment (both doses), additional patients achieved remission and LDA at each successive time point assessed through wk 24 in both studies; some additional patients achieved remission and LDA between wks 24 and 52 in MOBILITY. Normalization of physical function (HAQ-DI <0.5) was evident in both sarilumab-treated groups in MOBILITY by wk 12 (nominal P<0.05 vs placebo, both doses); a slight increase in the number of patients achieving normalization was observed by wk 52. In TARGET, a small numerical difference in HAQ-DI normalization was seen in favor of sarilumab treatment at all time points even though this endpoint did not achieve nominal significance.
Conclusion: In MTX-IR and TNF-IR patients with RA treated with sarilumab, efficacy became evident as early as wk 4. The numbers of patients who achieved remission or LDA increased through wk 24 with ongoing sarilumab treatment in both studies, with some further increases observed up to wk 52 in MOBILITY.
References:
1. Fleischmann et al. RMD Open. 2017;3:e000382.
2. Fleischmann et al. Ann Rheum Dis. 2015;74:1132-1137.
3. Hirata et al. Arthritis Res Ther. 2013;15:R135.
To cite this abstract in AMA style:
Genovese MC, Mangan EK, Fay J, Kimura T, van Hoogstraten H, Fleischmann R. Improvements in Remission and Low Disease Activity Are Achieved with Ongoing Sarilumab Treatment, in Patients with Rheumatoid Arthritis in 2 Phase 3 Studies [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/improvements-in-remission-and-low-disease-activity-are-achieved-with-ongoing-sarilumab-treatment-in-patients-with-rheumatoid-arthritis-in-2-phase-3-studies/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvements-in-remission-and-low-disease-activity-are-achieved-with-ongoing-sarilumab-treatment-in-patients-with-rheumatoid-arthritis-in-2-phase-3-studies/