Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In the randomized, phase 3, GO-VIBRANT study, more patients with psoriatic arthritis (PsA) achieved ACR 20/50/70 after 24 weeks IV treatment with the anti-TNFa monoclonal antibody golimumab (GLM-IV) than placebo (PBO) (p< 0.001). After cross-over from PBO to GLM-IV at week 24, 52-week achievement of ACR responses was similar between the two treatment groups.1 Here we examine effects on measures of health-related quality of life (HRQoL) for up to 52 weeks of treatment.
Methods: Adult patients with active PsA who met CASPAR criteria (N=480) were randomized (1:1) to GLM-IV 2 mg/kg at weeks 0, 4, then every 8 weeks or matching PBO through week 20 then cross-over to GLM-IV at weeks 24, 28, then every 8 weeks. Physical function was assessed using the Health Assessment Questionnaire-Disability Index (HAQ-DI). Measures of HRQoL included Short-Form-36 Physical and Mental Component Summaries (SF-36 PCS/MCS), Functional Assessment of Chronic Illness Therapy (FACIT)-Fatigue, EuroQol-5D visual analog scale (EQ-VAS), and Dermatology Life Quality Index (DLQI), assessed at weeks 0, 8, 14, 24, 36, and 52.
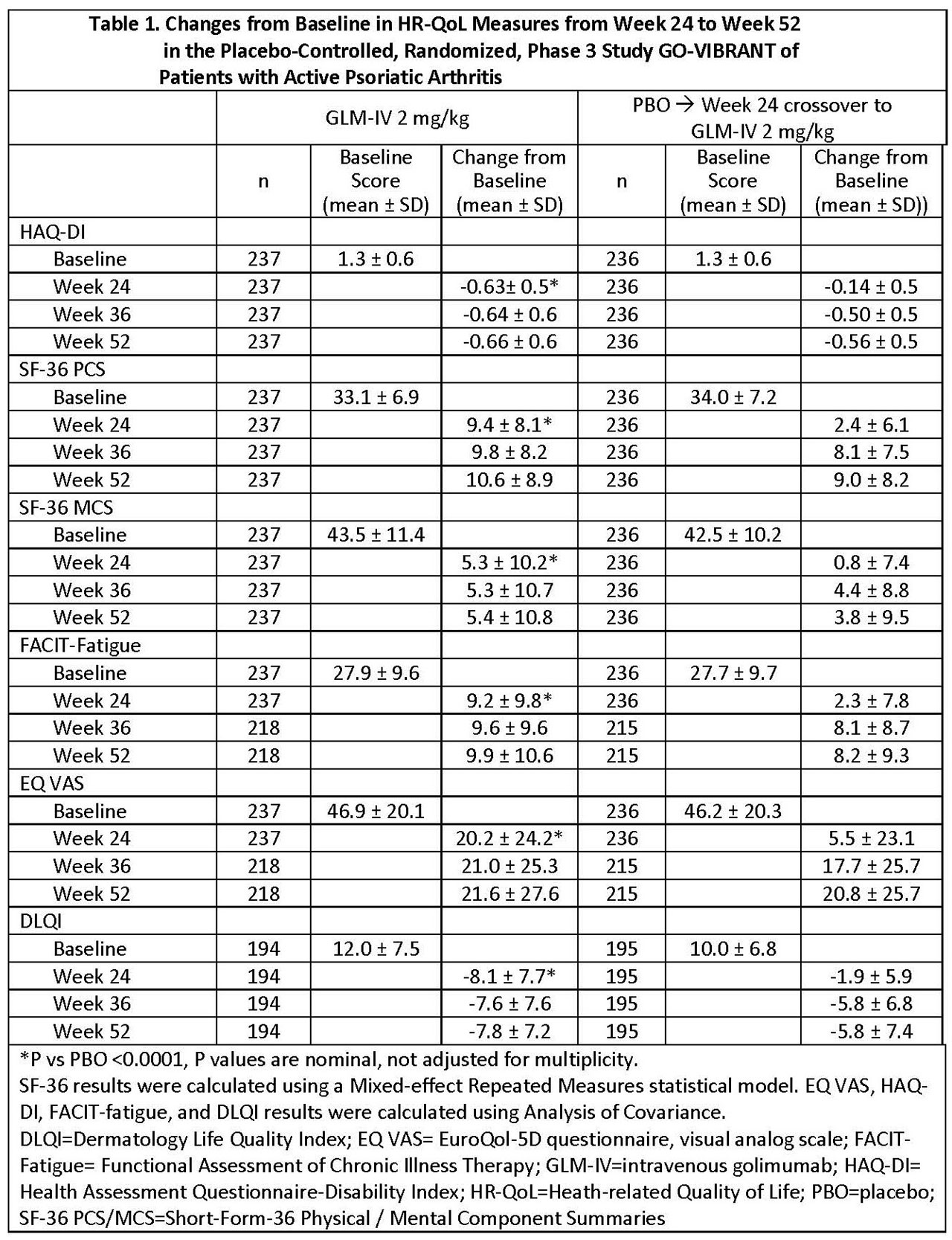
Results: GLM- IV and PBO groups had comparable HRQoL characteristics at baseline (Table 1). At 24 weeks, changes from baseline were greater for GLM-IV vs PBO, respectively (HAQ-DI, -0.63 vs -0.14; SF-36 PCS, 9.4 vs 2.4; SF-36 MCS, 5.3 vs 0.8; FACIT-Fatigue, 9.2 vs 2.3; EQ-VAS, 20.2 vs 5.5; and DLQI, -8.1 vs -1.9). At week 24 more patients receiving GLM-IV than PBO achieved minimal clinically important improvements from baseline in HAQ (≥0.35 points),2 SF-36 (≥5points),3 and FACIT-fatigue (≥4 points).4 Among patients randomized to GLM-IV, changes in HRQoL measures were maintained from week 24 to week 52. Among patients randomized to PBO, after switching to GLM-IV at week 24, improvements in HRQoL measures from week 36 to week 52 were comparable to those of patients originally randomized to GLM-IV (Tables 1 and 2).
Conclusion: Improvements in HRQoL among patients with PsA after 24 weeks’ GLM-IV treatment were greater than PBO and were maintained through week 52 of treatment. Patients switching from PBO to GLM-IV at week 24 experienced improvements in HRQoL by week 36, which were maintained through week 52 and were similar to those achieved by patients originally randomized to GLM-IV.
- Husni et al. Arthritis Care Res. 2019 DOI:10.1002/acr.23905.
- Mease et al. J Rheumatol. 2011;38:2461.et al.
- Lubeck et al. 2004;22:27.
- Cella et al. J Rheumatol. 2005;32:811.
To cite this abstract in AMA style:
Husni M, Harrison D, Hsia E, Chan E, Han C, Kafka S, Lo K, Kim L, Kavanaugh A. Improvements in Health-Related Quality of Life in Psoriatic Arthritis Patients Treated with Intravenous Golimumab, an Anti-TNFα Monoclonal Antibody: 1-Year Results of a Phase III Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/improvements-in-health-related-quality-of-life-in-psoriatic-arthritis-patients-treated-with-intravenous-golimumab-an-anti-tnf%ce%b1-monoclonal-antibody-1-year-results-of-a-phase-iii-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvements-in-health-related-quality-of-life-in-psoriatic-arthritis-patients-treated-with-intravenous-golimumab-an-anti-tnf%ce%b1-monoclonal-antibody-1-year-results-of-a-phase-iii-trial/