Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: In rheumatoid arthritis (RA), the proinflammatory cytokine IL-6 is associated with mental disorders such as depression and anxiety.1 Furthermore, depression and anxiety are comorbidities associated with a lower response to RA therapy.2
In this interim analysis (data cut-off 1 Feb 2019) of the non-interventional study ARATA, we analyzed the possible influence of depressiveness and anxiety on the effectiveness of tocilizumab (RoActemra®) therapy for the first time
Methods: The ARATA study (NCT02251860) examined the effectiveness and tolerability of subcutaneous tocilizumab therapy in patients with RA in routine practice for up to 104 weeks. Patients were divided into subgroups according to Beck Depression Inventory (BDI)-II or State-Trait Inventory (STAI-X2) category at baseline.
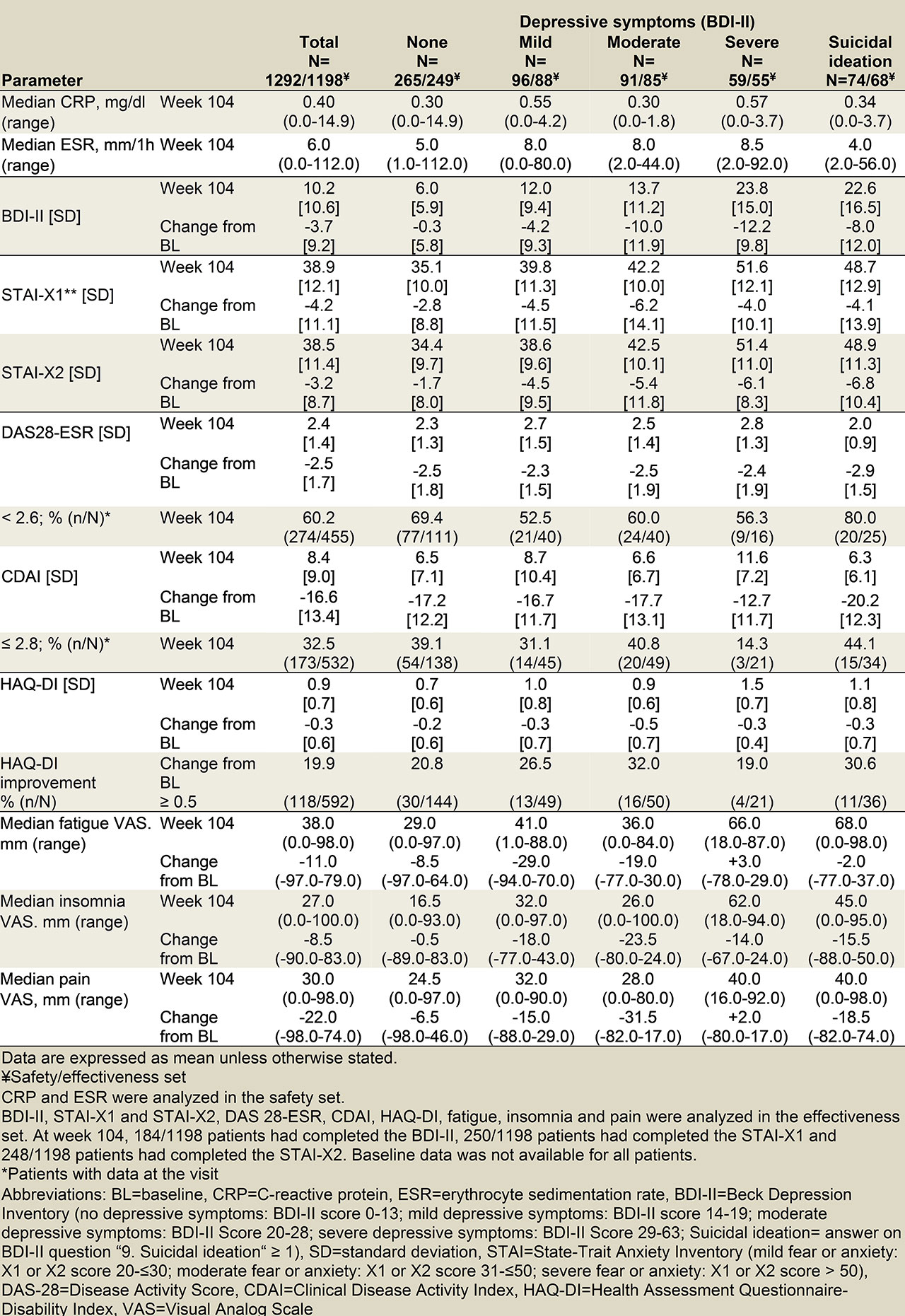
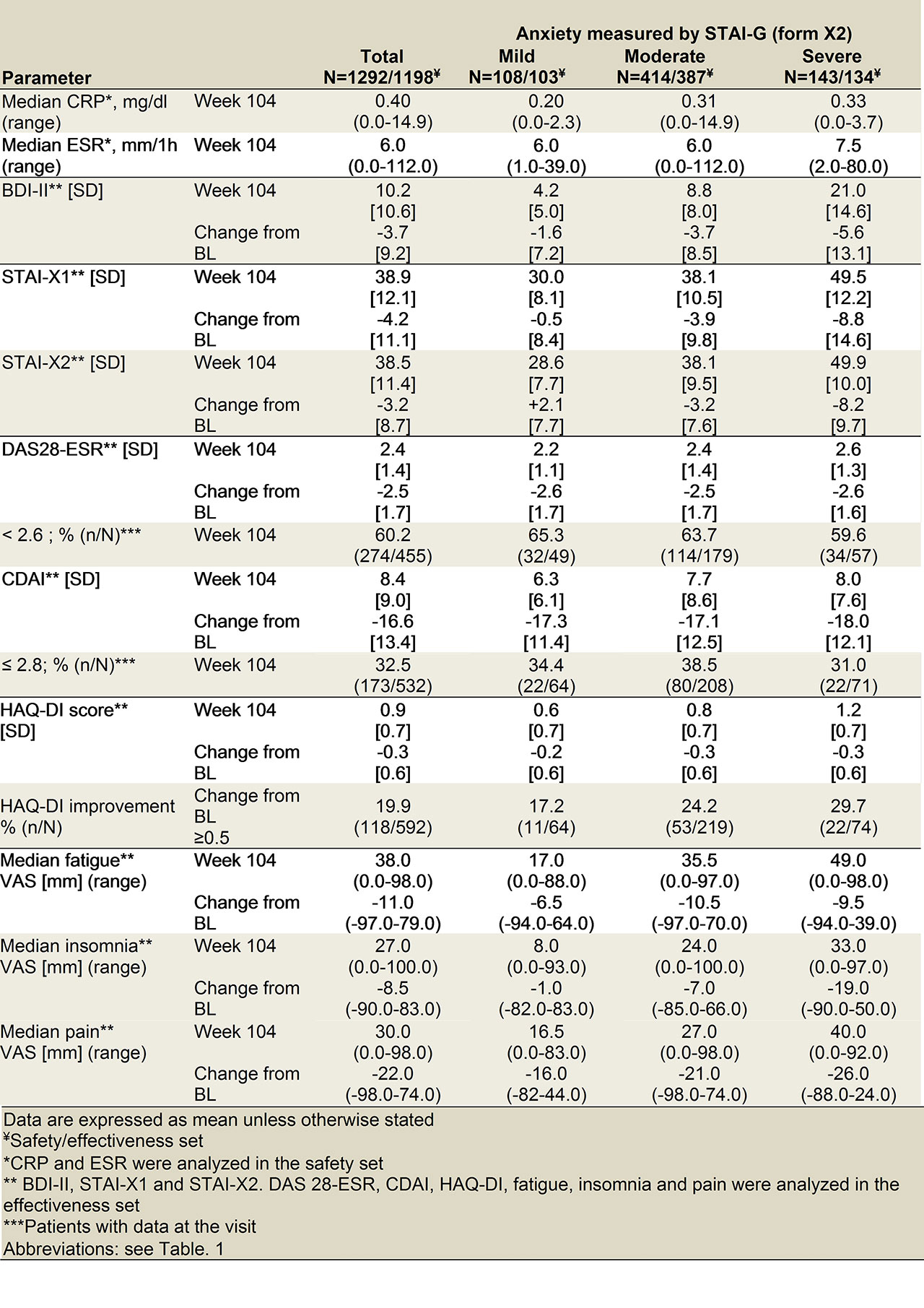
Results: The safety and effectiveness sets were comprised of 627 and 574 patients, respectively. Patients in all depressive symptom and anxiety categories benefitted from tocilizumab therapy (tables 1 and 2), regardless of baseline characteristics. Patients had an overall reduction in depressiveness, and a slight reduction of fear and anxiety in all depressiveness categories. During tocilizumab treatment, patients with positive answers to the question about suicidal ideation benefitted the most in regards to disease activity outcomes (DAS28-ESR, CDAI), while patients with severe depressive symptoms benefitted the least. The more severe anxiety categories had greater proportions of patients with a HAQ-DI improvement of at least 0.5 at week 104.
Results in patients-reported outcomes mirrored the results in effectiveness outcomes. Patients without, with mild or with moderate depressive symptoms and patients with positive answers to the question about suicidal ideation had reductions in fatigue, insomnia and pain. In contrast to insomnia, patients with severe depressive symptoms had slight increases in fatigue and pain.
Fatigue, insomnia, and pain were reduced in all three anxiety categories – the more severe the anxiety category, the greater the reductions were in insomnia and pain.
Adverse events were documented more often for patients with depressive symptoms and anxiety. However, there was no difference in serious adverse events. Discontinuation due to adverse events increased with more severe depressive symptoms and anxiety.
Conclusion: For the first time, the influence of depressiveness and anxiety on TCZ effectiveness was investigated in a German collective of RA patients in routine practice. The interim analysis of the non-interventional ARATA study underlines not only the clinical effectiveness and safety, but also a positive influence of the therapy on depressiveness, anxiety, fear, fatigue, insomnia and pain. Patients with severe depression did not benefit from fatigue and pain despite improved disease activity.
- Choy & Calabrese, Rheumatology (Oxford). 2018, 57(11):1885-1895.
- Matcham et al., Rheumatology (Oxford) 2016; 55(2):268-278.
To cite this abstract in AMA style:
Behrens F, Biewer W, Burmester G, Feuchtenberger M, Hofmann M, Kästner P, Kellner H, König R, Liebhaber A, Luig C, Max R, Sternad P, Tony H, Amberger C. Improvement of Mental Health and Quality of Life During Therapy with Tocilizumab [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/improvement-of-mental-health-and-quality-of-life-during-therapy-with-tocilizumab/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-of-mental-health-and-quality-of-life-during-therapy-with-tocilizumab/