Session Information
Date: Monday, October 22, 2018
Title: Spondyloarthritis Including Psoriatic Arthritis – Clinical Poster II: Clinical/Epidemiology Studies
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Stiffness is an important component of inflammatory arthritis and plays a role in PsA flare. Patients with inflammatory arthritis report difficulty with activities, “slowing down” due to stiffness, and reduced quality of life. Stiffness is hard to quantify because it cannot be measured directly. We examined morning stiffness in PsA patients treated with apremilast (APR) for 16 weeks in the ACTIVE study and explored the relationship between improvements in morning stiffness and pain, physical function, and Patient’s Global Assessment of Disease Activity (PtGA).
Methods: Subjects who met CASPAR criteria for PsA, were biologic-naïve, and had prior exposure to ≤1 conventional disease-modifying anti-rheumatic drug (DMARD) were randomized (1:1) to APR 30 mg BID or placebo (PBO) for 24 weeks; thereafter, all subjects received active treatment with APR. In the post-hoc analysis, changes in morning stiffness severity and effect on pain, physical function, and PtGA from baseline (BL) to Week 16 were compared between treatment arms. Duration of morning stiffness and severity, assessed by subjects’ reported categories of stiffness (none, mild, moderate, moderately severe, severe), were reported. An ANCOVA model adjusting the BL value was used in the analysis, where missing values were imputed using the last-observation-carried-forward approach.
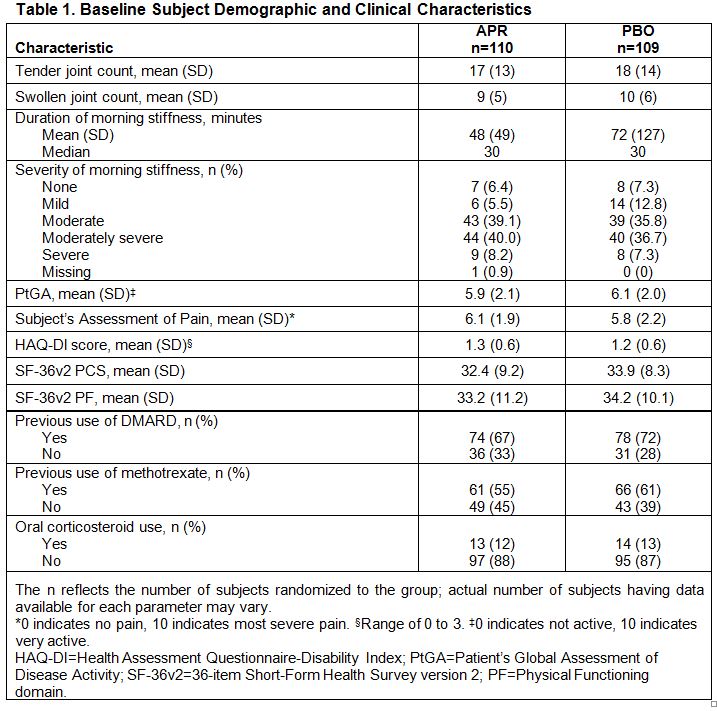
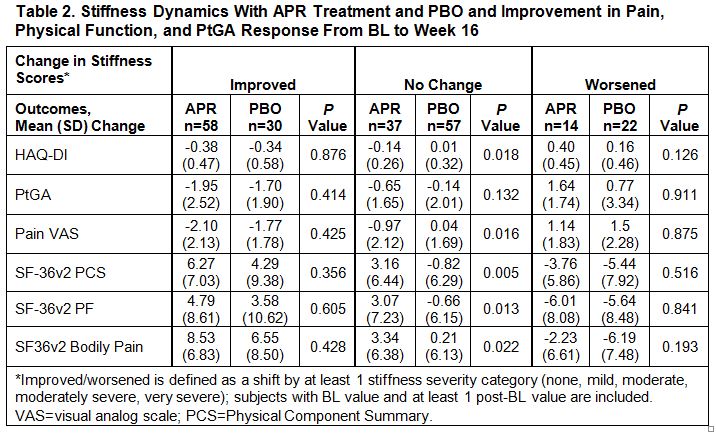
Results: A total of 219 subjects were randomized (APR: n=110; PBO: n=109), and 192 remained in the study through Week 16. Most subjects (84%) reported moderate to severe morning stiffness at BL (Table 1). As early as Week 2, a significantly greater proportion of APR vs. PBO subjects experienced morning stiffness severity improvements (≥1 category improvement) (43% vs. 21%; P=0.0007). Significant improvements in morning stiffness severity were sustained through Week 16 in APR vs. PBO subjects (46% vs. 26%; P=0.0015). Subjects with improvements in morning stiffness severity in the APR and PBO groups showed consistent improvements at the group level in pain, physical function, and PtGA at Week 16 (Table 2). In patients with unchanged stiffness severity, greater improvements were observed across outcome measures in the APR vs. PBO group, although to a lesser extent compared with the improved stiffness group. Worsened morning stiffness was associated with lack of improvement in other patient-reported outcome measures.
Conclusion: In biologic-naïve subjects with PsA treated with APR for 16 weeks, improvement in morning stiffness severity was evident as early as Week 2 and associated with improvements in pain, physical function, and PtGA response.
To cite this abstract in AMA style:
Orbai AM, Walsh J, Nash P, Teng L, Guerette B, Alten R. Improvement in Morning Stiffness in Subjects with Psa Is Associated with Improvements in Pain, Physical Function, and Patient Global Response to Treatment [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/improvement-in-morning-stiffness-in-subjects-with-psa-is-associated-with-improvements-in-pain-physical-function-and-patient-global-response-to-treatment/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-in-morning-stiffness-in-subjects-with-psa-is-associated-with-improvements-in-pain-physical-function-and-patient-global-response-to-treatment/