Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Invasive pneumococcal
disease is approximately four times more common among patients with rheumatoid
arthritis (RA) and systemic lupus erythematosus (SLE) compared to healthy
counterparts. In 2014, the Advisory Committee on Immunization Practices issued
a new guideline recommending vaccination against pneumococcal organisms with the
pneumococcal conjugate vaccine (PCV13) for all immunosuppressed patients,
regardless of age, with the goal of reducing the rate of serious infections and
mortality among high-risk patients. Our goal was to increase the rate of PCV13
vaccinations in our immunosuppressed patients at an academic VA medical center.
Methods:
Plan-Do-Study-Act
(PDSA) methodology was used over two cycles within a single clinic.
Immunosuppressed patients were identified as those receiving any disease
modifying antirheumatic drugs (DMARDs), biologic
agents, or prednisone (dose > 10mg daily). PDSA Cycle 1 included (1) an
investigator-led educational session for providers and (2) making the PCV13
vaccine available in the clinic. PDSA Cycle 2 involved the investigators
identifying patients eligible for the PCV13 vaccine ahead of their clinic visit
and pending the PCV13 order in the electronic medical record. Receipt of the vaccine
was assessed via review of the electronic medication administration record. Provider
recommendation of the vaccine was assessed by review of the clinical note. Reasons
for patient refusal or lack of vaccine administration after provider
recommendation were not consistently available.
Results:
Six
rheumatology fellows and five faculty members participated in the quality
improvement activity. Prior to the intervention, zero patients received a PCV13
vaccine in clinic as they were not available, with
less than five patients receiving it outside of the clinic. PDSA Cycle 1 was 8
weeks long with 109 patients who were eligible for vaccination. 57 (52%) were
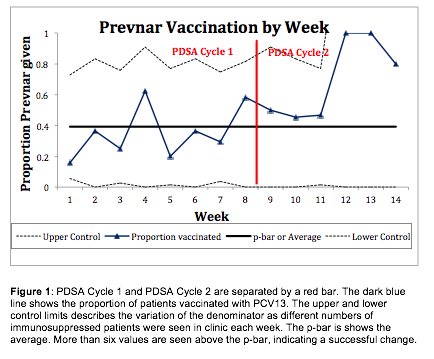
recommended to have the vaccine and 35 (32%) received it. PDSA Cycle 2 was 6
weeks long with 44 patients who were eligible for vaccination, 36 (82%) were recommended
to have the vaccine and 25 (57%) vaccinated (see Figure 1).
Conclusion:
One quality
improvement lecture updating providers on new
vaccination recommendations and availability of the vaccine can improve
vaccination patterns amongst immunosuppressed patients. Identifying patients in
need of vaccination prior to their clinic visit can improve vaccination rates
even further.
To cite this abstract in AMA style:
Bays A, Nayak R, Daikh DI, Yazdany J, Schmajuk G. Implementation of New Pneumococcal Vaccination Recommendations in an Academic Rheumatology Clinic [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/implementation-of-new-pneumococcal-vaccination-recommendations-in-an-academic-rheumatology-clinic/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/implementation-of-new-pneumococcal-vaccination-recommendations-in-an-academic-rheumatology-clinic/