Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: Patient Outcomes, Preferences, & Attitudes II: Patient Experience
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
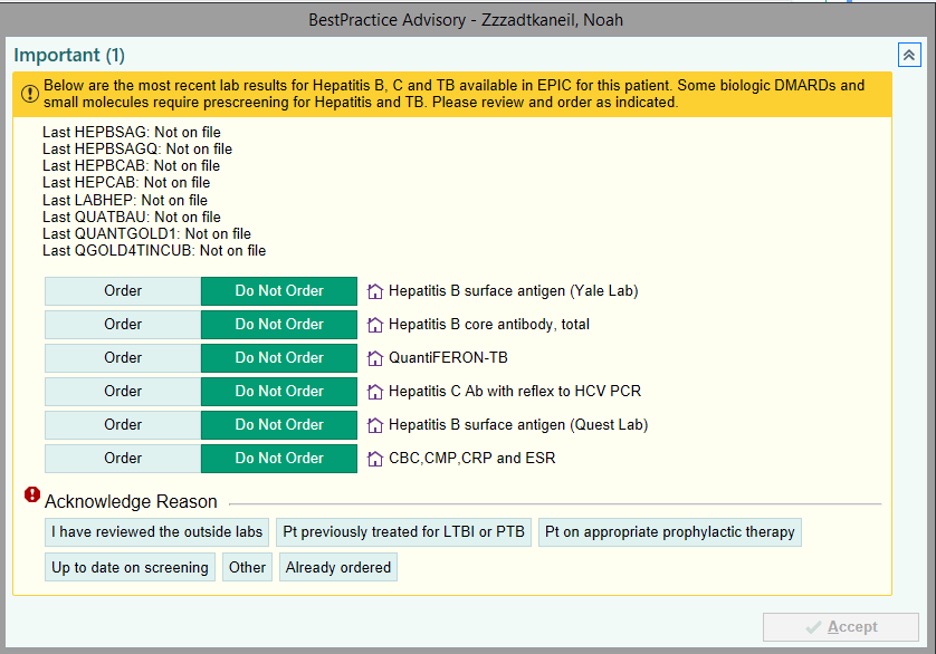
Background/Purpose: Biologic and targeted synthetic DMARDs (b/tsDMARDs) are widely used to treat patients with various autoimmune inflammatory diseases (ARD). Use of a b/tsDMARD in patients with pre-existing tuberculosis (TB), hepatitis B (HBV), or hepatitis C (HCV) can lead to significant morbidity and mortality. Many national and international societies recommend screening for one or all of these exposures prior to initiation of certain b/tsDMARDs. However, recommended timing intervals of screening are not clear and adherence to these recommendations varies widely. This quality improvement initiative focused on improving screening for TB, HBV, and HCV prior to new prescription of a b/tsDMARD using a best practice advisory (BPA) in the electronic health records (EHR).
Methods: Patients aged 18 years or older with at least one visit with a clinician (attending, fellow, or APRN) in the section of Rheumatology in the designated time frame were included. Upon a new prescription of a b/tsDMARD, clinicians were alerted via a BPA pop-up in the EHR about the last available results if any, for TB, HBV, and HCV, and allowed for quick ordering as appropriate. This BPA was implemented on December 1, 2020. Baseline screening proportions for TB (QuantiFERON), HBV, and HCV for patients prescribed a new b/tsDMARD from October 1, 2017 to November 30, 2020 (pre-BPA period) were compared with those of patients with prescriptions from December 1, 2020 to March 3, 2022 (post-BPA period).
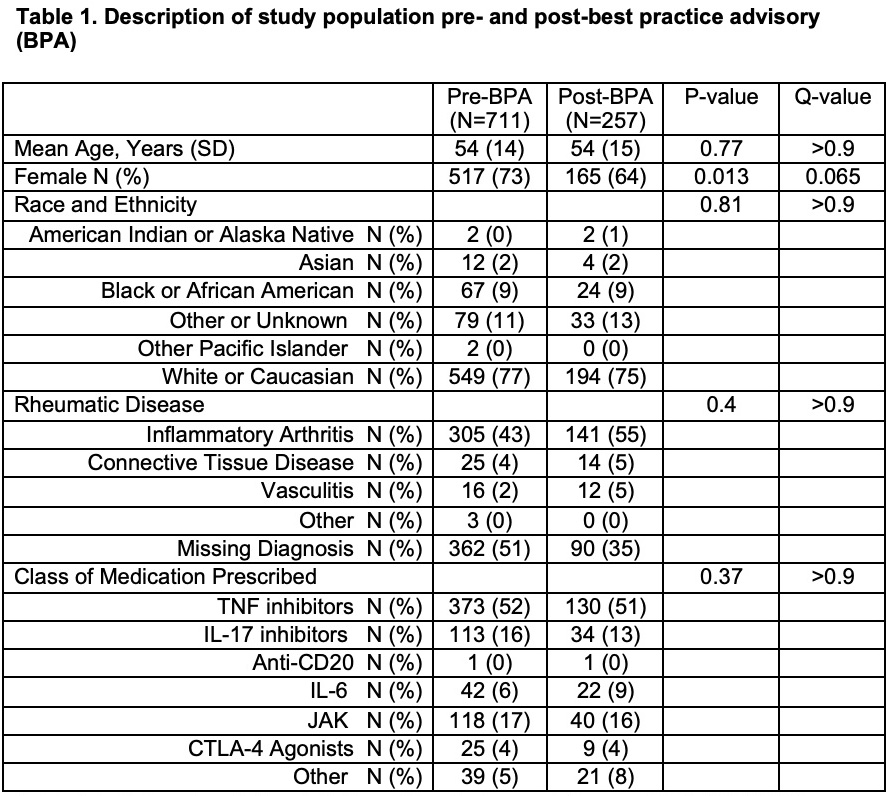
Results: A total of 711 patients pre-BPA and 257 patients post-BPA implementation were included in the study. The BPA implementation was associated with statistically significant improvement in screening for TB, HBsAg, HBcAb, and HCV Ab or HCV viral load (Table 1). Partial hepatitis B testing (screening for HBcAb or HBsAg) was significantly changed by the BPA (pre-BPA 364 of 711 (51%) vs post-BPA 186 of 257 (72%), p=< 0.001). Complete hepatitis B testing (screening for both HBcAb and HBsAg) also improved after BPA implementation (pre-BPA 222 of 711 (31%) vs post-BPA 127 of 257 (49%), p< 0.001). A total of 154 patients (22%) in the pre-BPA and 117 patients (46%) in the post-BPA periods were appropriately screened for all three infectious diseases, which was statistically significant (p< 0.001). Multivariable logistic analysis revealed in the pre-BPA period, relative to attending physicians, fellows were significantly more likely to order testing (HBcAb p=< 0.001, HBsAb p=< 0.001, HBsAg p=0.005, partial Hep B testing p=0.006, complete Hep B testing p=< 0.001, HCV Ab or HCV RNA p=0.002, all four tests p=< 0.001) after accounting for multiple comparisons. In the pre-BPA period, each year of age decreased the adjusted odds of TB testing by 2% (OR 0.98; 95% CI 0.97, 1.0; p=0.006) and males had lower adjusted odds for HBcAb testing than females (OR 0.58; 95% CI 0.39, 0.85). In the post-BPA period, there were no statistically significant findings for any patient or clinician variable.
Conclusion: Implementation of a BPA in the EHR can improve infectious disease screening for patients with ARD initiating b/tsDMARDs and has potential to improve patient safety and eliminate screening discrepancies.
*Q-value=<0.001
**Q-value=>0.9
To cite this abstract in AMA style:
Baker H, Fine R, Suter F, Allore H, Hsiao B, Chowdhary V, Lavelle E, Chen P, Hintz R, Suter L, Danve A. Implementation of a Best Practice Advisory to Improve Infection Screening Prior to New Prescriptions of Biologics and Targeted Synthetic Drugs [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/implementation-of-a-best-practice-advisory-to-improve-infection-screening-prior-to-new-prescriptions-of-biologics-and-targeted-synthetic-drugs/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/implementation-of-a-best-practice-advisory-to-improve-infection-screening-prior-to-new-prescriptions-of-biologics-and-targeted-synthetic-drugs/