Session Information
Date: Sunday, November 17, 2024
Title: Vasculitis – Non-ANCA-Associated & Related Disorders Poster II
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Giant cell arteritis (GCA) is both a medical emergency and diagnostic challenge. Up to 45% of patients may require emergency department (ED) visits and/or hospitalization to expedite investigations. This leads to significant health care utilization and cost. This study aims to determine if a Fast-Track referral algorithm, combined with a non-invasive diagnostic tool, can reduce diagnostic delays, ED visits, hospitalizations, and visual loss associated with GCA.
Methods: A prospective study was conducted in our GCA Fast-Track (FT) clinic from June to September 2022. Patients referred to the FT clinic underwent an initial assessment and blood work performed within 24 hours of referral. A GCA expert evaluated the patients in the next 24-72 hours and performed a Color Doppler ultrasound (CDUS) of the temporal and axillary arteries. Consecutive patients with new-onset GCA confirmed through our FT clinic were included (FT group).
The control group was a retrospective cohort of patients with GCA (2003-2016) who had a temporal artery biopsy performed prior to the FT clinic implementation.
Collected information included the delay from 1) the first GCA symptom to glucocorticoids (GCs) initiation, 2) the first GCA symptom to the final diagnosis, and 3) the first medical contact to the final diagnosis. The occurrences of permanent vision loss, ED visits and hospitalizations (including their durations) were also recorded. Descriptive statistics for both the FT group and the control group were computed using R (version 4.2.1).
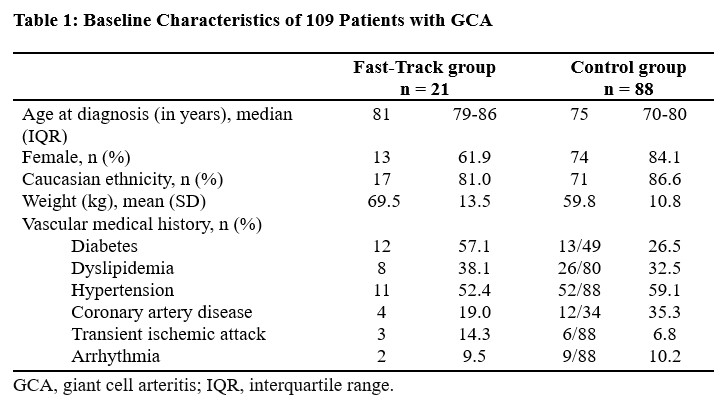
Results: A total of 109 patients with GCA were included: 21 patients in the FT group and 88 patients in the control group (Table 1). All patients with GCA met the ACR classification criteria for GCA (1990 or 2022 version).
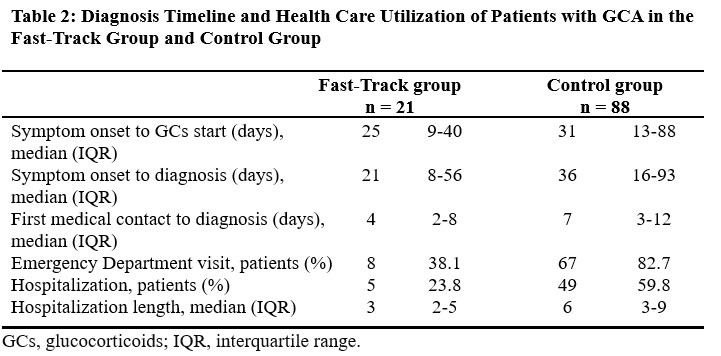
The delay from GCA symptom onset to GCs was 25 days (interquartile range [IQR] 9 to 40 days) vs. 31 days (IQR 13 to 88 days) for the FT and control group, respectively (Table 2). The delay from GCA symptom onset to the final diagnosis of GCA was 21 days (IQR 8 to 56 days) in the FT group and 36 days (IQR 16 to 93 days) in the control group. The time elapsed from the first medical contact to the final diagnosis of GCA was 4 days (IQR 2 to 8 days) for the FT group and 7 days (IQR 3 to 12 days) for the control group.
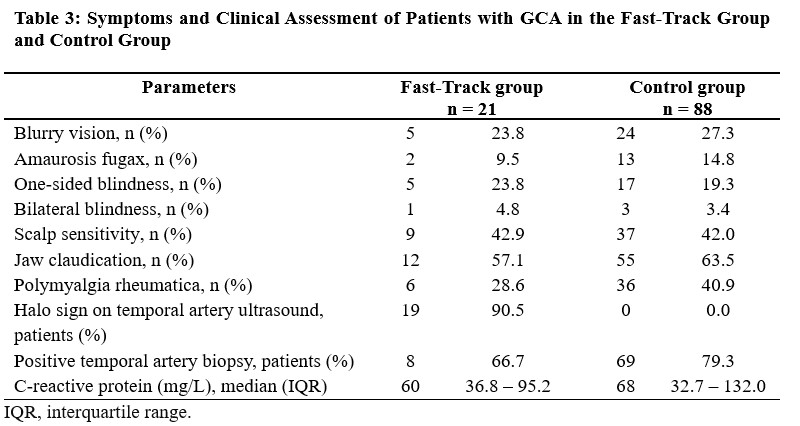
ED visits occurred in 38.1% of patients in the FT group and 82.7% of patients in the control group. Hospitalization to investigate GCA occurred in 23.8% of patients of the FT group (median duration 3 days, IQR = 2 to 5 days) compared to 59.8% of patients in the control group (median duration 6 days, IQR = 3 to 9 days). The proportion of unilateral vision loss at diagnosis was 23.8% in the FT group and 19.3% in the control group (Table 3).
Conclusion: Implementing a GCA Fast-Track referral method with a bedside non-invasive diagnostic test such as CDUS can reduce health care utilization in GCA. The delay from the onset of GCA symptoms to first GCs was high in both groups and might explain the similar rates of vision loss at diagnosis. The effect of a GCA educational and awareness program in the general population to reduce incident vision loss should be investigated.
To cite this abstract in AMA style:
Dhahbi A, Ducharme-Benard S, Hussein S, Meunier R, Baalbaki H, Ross C, Makhzoum J. Impacts of a Fast-Track Referral Clinic on Health Care Utilization, Delays and Complications in Patients with Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impacts-of-a-fast-track-referral-clinic-on-health-care-utilization-delays-and-complications-in-patients-with-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impacts-of-a-fast-track-referral-clinic-on-health-care-utilization-delays-and-complications-in-patients-with-giant-cell-arteritis/