Session Information
Date: Sunday, November 13, 2022
Title: RA – Treatment Poster III
Session Type: Poster Session C
Session Time: 1:00PM-3:00PM
Background/Purpose: The phase 3 SELECT-CHOICE trial of patients with rheumatoid arthritis (RA) and prior inadequate response to biologic DMARD(s) (bDMARD-IR) demonstrated superiority of the JAK inhibitor upadacitinib (UPA) vs abatacept (ABA) in the mean change from baseline (BL) in DAS28(CRP) and in the proportion achieving DAS28(CRP) < 2.6 at week (wk) 12, with higher incidence of serious adverse events reported in the UPA treatment group.
We evaluate the impact of UPA vs ABA on individual components of composite measures of disease activity in SELECT-CHOICE.
Methods: In SELECT-CHOICE, a double-blind phase 3 trial, bDMARD-IR patients were randomly assigned to UPA 15 mg once daily or ABA, each with background conventional synthetic DMARDs, for 24 wks. For this post hoc analysis, the proportions of patients achieving improvement from BL through wk 24 in ACR core variables (including SJC, TJC, Patient Global Assessment [PtGA], Physician Global Assessment [PhGA], pain, HAQ-DI, and hsCRP) and Boolean remission criteria were evaluated. Differences in the cumulative distributions of CDAI, DAS28(hsCRP), SDAI, and ACR-n (the lowest of percent change in TJC, percent change in SJC, or median of the other 5 ACR components) were determined using the Kolmogorov-Smirnov test and are reported as observed. For all other variables, non-responder imputation was applied for missing data. Nominal P values are provided throughout.
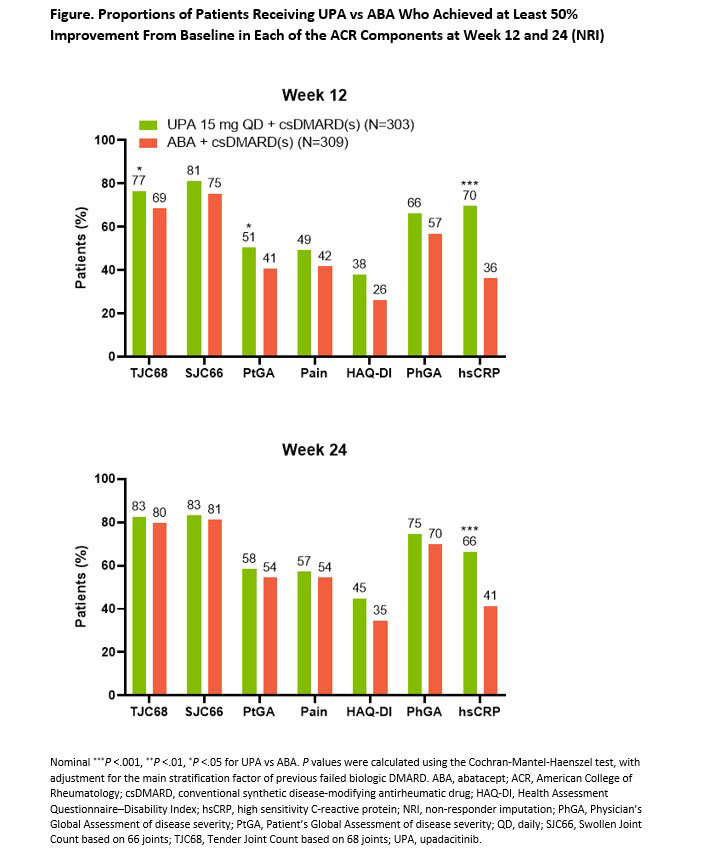
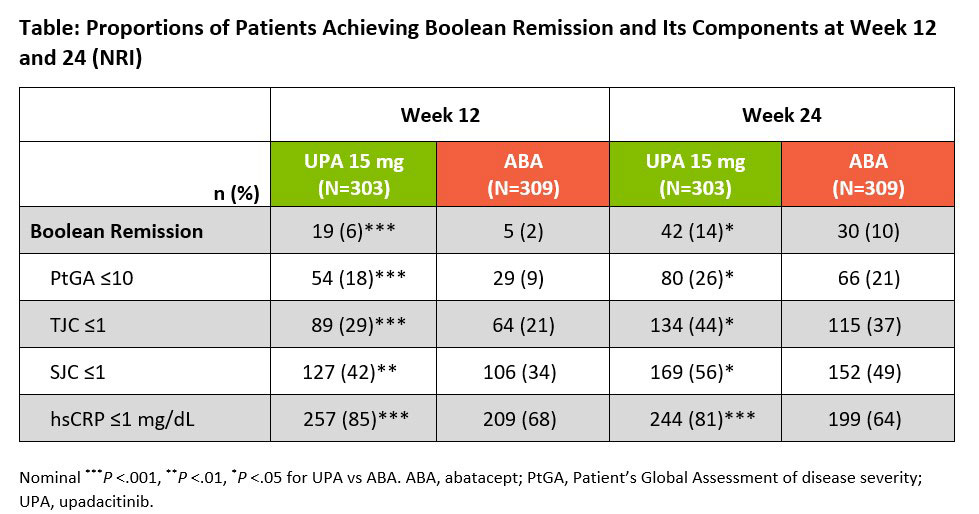
Results: A total of 616 bDMARD-IR patients with moderate to severe RA were randomized in SELECT-CHOICE (UPA 15 mg, n=303; ABA, n=309). BL demographic and disease characteristics were generally comparable between treatment groups, with a mean disease duration of approximately 12 years and mean CDAI of 39.6. At wk 12, more patients receiving UPA vs ABA achieved ≥50% improvements from BL in TJC68, PtGA, and hsCRP, with comparable proportions observed between UPA and ABA for the remaining ACR components (Figure). At wk 24, similar proportions of patients receiving UPA and ABA achieved ≥50% improvements in all but the hsCRP component. Overall, 15% and 26% of patients on UPA compared with 6% and 15% on ABA demonstrated ≥50% improvements across all ACR components at wks 12 and 24, respectively. At wks 12 and 24, Boolean remission was achieved by 6% and 14% of patients on UPA vs 2% and 10% of patients on ABA, respectively; the proportion of patients in both treatment groups achieving the individual Boolean components were also reported (Table). While comparable at BL, cumulative distributions of CDAI, SDAI, DAS28(hsCRP), and ACR-n were improved on UPA vs ABA at wk 12 (all nominal P < 0.05); differences persisted for most measures at wk 24.
Conclusion: In this post hoc analysis of bDMARD-IR RA patients, improvements in components of disease measures were reported for both UPA and ABA through 24 weeks, with numeric differences noted for several components. Nominally higher attainment of Boolean remission and its components were observed for UPA over ABA.
References
1. Rubbert-Roth A, et al. N Engl J Med 2020; 383:1511-21.
To cite this abstract in AMA style:
van Vollenhoven R, Rubbert-Roth A, Hall S, Xavier R, Shmagel A, Song Y, Anyanwu S, Strand V. Impact of Upadacitinib versus Abatacept on Individual Disease Outcomes in Patients with Rheumatoid Arthritis and Inadequate Responses to Biologic DMARDs [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/impact-of-upadacitinib-versus-abatacept-on-individual-disease-outcomes-in-patients-with-rheumatoid-arthritis-and-inadequate-responses-to-biologic-dmards/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-upadacitinib-versus-abatacept-on-individual-disease-outcomes-in-patients-with-rheumatoid-arthritis-and-inadequate-responses-to-biologic-dmards/