Session Information
Date: Sunday, November 12, 2023
Title: (0510–0542) Spondyloarthritis Including Psoriatic Arthritis – Treatment: AxSpA Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Physical activity is associated with reduced pain, improved mobility and physical function in people with ankylosing spondylitis (AS) and plays a crucial role in AS management. Despite this, the impact of pharmacologic interventions on physical activity is rarely measured or reported in clinical research. Wearable technology enables passive collection of objective physical activity data. We previously demonstrated high levels of wearable device adherence in patients with active AS in the SELECT-AXIS 2 phase 3 trial and reported this cohort’s baseline physical activity patterns.1 Here, we evaluated the effect of upadacitinib (UPA) vs placebo (PBO) on physical activity in this patient cohort through 14 weeks (wks).
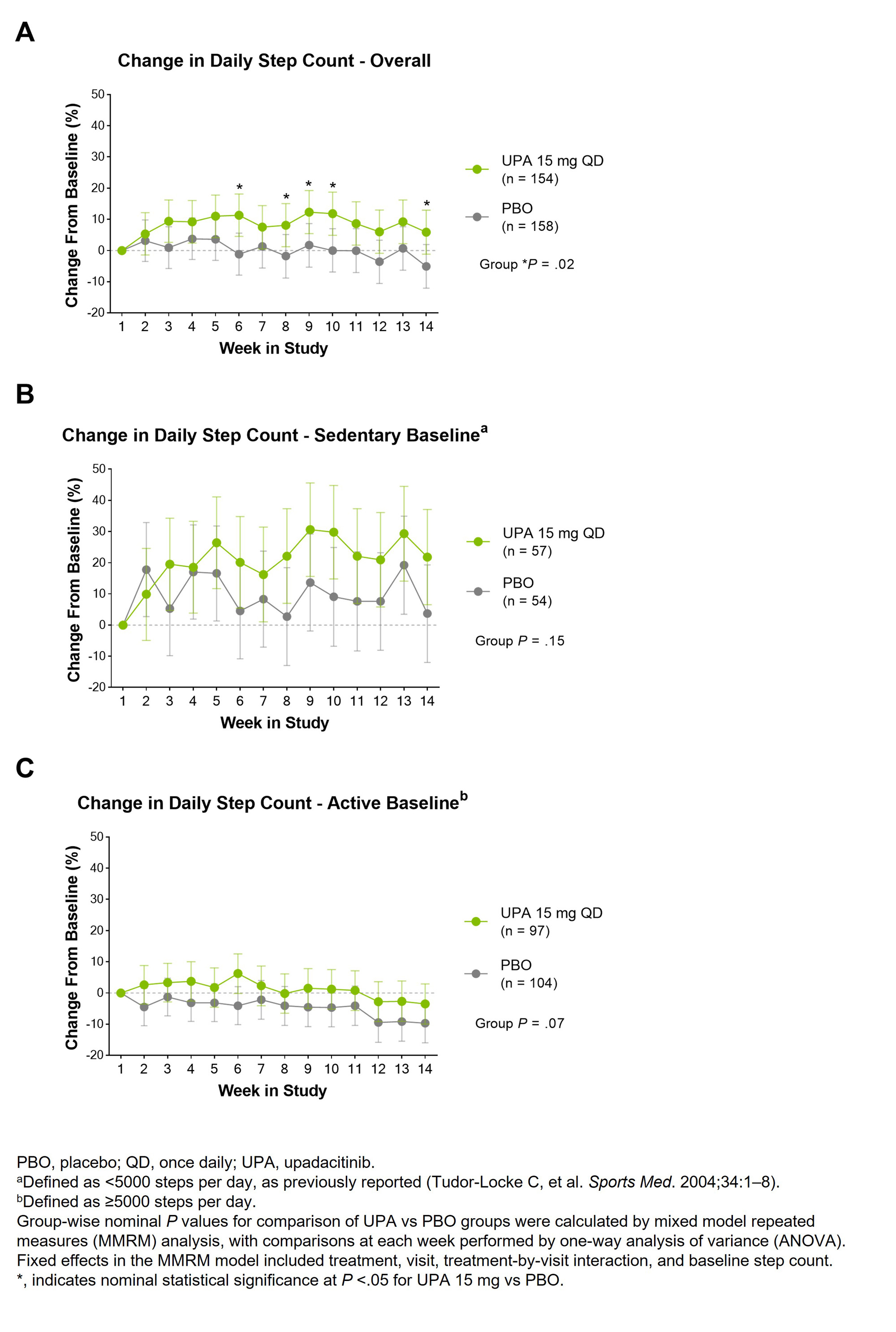
Methods: Patients with active AS with an inadequate response or intolerance to biologic DMARDs (bDMARD-IR) were randomized to receive UPA 15 mg once daily or PBO.2 As part of the study design, patients were required to wear a medical-grade wrist-worn actigraphy device during the 14-wk, PBO-controlled portion of the study. For inclusion in physical activity analyses, patients needed to have at least 3 adherent days (defined as 16 hrs per day of device usage) out of the first 7 days after trial entry. The percent change from the first wk of the study (defined as baseline) in mean daily steps was evaluated over 14 wks. Changes in physical activity were also assessed in the subsets of patients who demonstrated an active (≥5000 steps per day per day) or sedentary lifestyle (< 5000 steps per day) at baseline. Group-wise nominal P values for comparison of UPA vs PBO groups through 14 wks were calculated by mixed model repeated measures analysis, with comparisons at each wk performed by one-way analysis of variance.
Results: Of 420 total patients, physical activity data were collected from 394 participants, and 312 (UPA, n=154; PBO, n=158) met minimal adherence criteria based on their first 7 days of study participation. In these patients, adherence was 83.5% of study days through 14 wks.1 Patients treated with UPA had numerically higher mean daily step counts than those treated with PBO, with an 11-percentage point improvement vs PBO at wk 14 (mean absolute difference of 345 steps per day; P < .05) (Fig 1A). In patients with a sedentary lifestyle at baseline, a 22% improvement in the mean daily step count was observed with UPA from baseline to wk 14 compared to a 4% improvement with PBO (mean absolute difference of 264 steps per day), although these differences were not significant (Fig 1B). Patients with an active lifestyle at baseline also showed numerically better maintenance of their daily step counts over 14 wks with UPA compared to PBO where step counts declined over time (Fig 1C).
Conclusion: In bDMARD-IR AS patients, UPA treatment led to numerically greater improvements compared to PBO in physical activity as measured by a wearable device over 14 wks, especially in sedentary patients. These findings support the utility of passively collected actigraphy measurements to monitor the effect of a targeted therapy on physical activity and further exploring possible heterogeneous effects on step count depending on baseline activity.
1. Mease et al. Ann Rheum Dis. 2023;82:626.
2. van der Heijde et al. Ann Rheum Dis. 2022;81:1515–23.
To cite this abstract in AMA style:
Curtis J, Grainger R, Webster D, Shen J, Biljan A, Shmagel A, Wung P, Mease P. Impact of Upadacitinib on Wearable Device-Measured Physical Activity in Patients with Ankylosing Spondylitis from the SELECT-AXIS 2 Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/impact-of-upadacitinib-on-wearable-device-measured-physical-activity-in-patients-with-ankylosing-spondylitis-from-the-select-axis-2-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-upadacitinib-on-wearable-device-measured-physical-activity-in-patients-with-ankylosing-spondylitis-from-the-select-axis-2-trial/