Session Information
Date: Monday, November 13, 2023
Title: (1412–1441) Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II: SpA
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Enthesitis and dactylitis are associated with reduced quality of life and greater impairment in daily activities. In the SELECT-PsA 2 phase 3 trial, the JAK inhibitor upadacitinib (UPA) demonstrated greater resolution of enthesitis and dactylitis compared to placebo (PBO) in patients with PsA. However, while the sites of enthesitis and dactylitis involvement are highly variable, relatively limited data are available regarding location-specific resolution of these disease manifestations in PsA. In this post hoc analysis, we evaluated site-specific responses of enthesitis and dactylitis to treatment with UPA over 56 weeks in patients with PsA from SELECT-PsA 2.
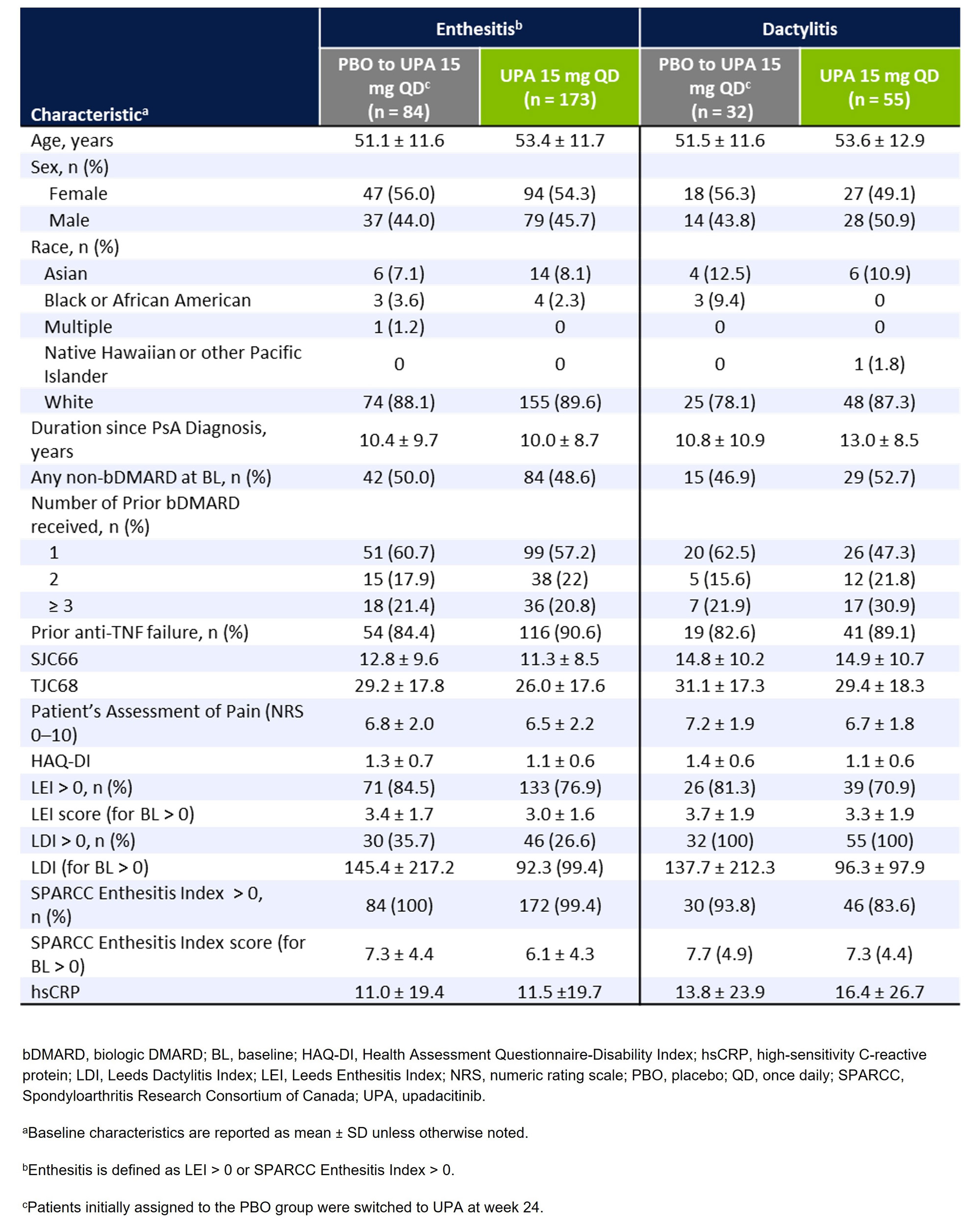
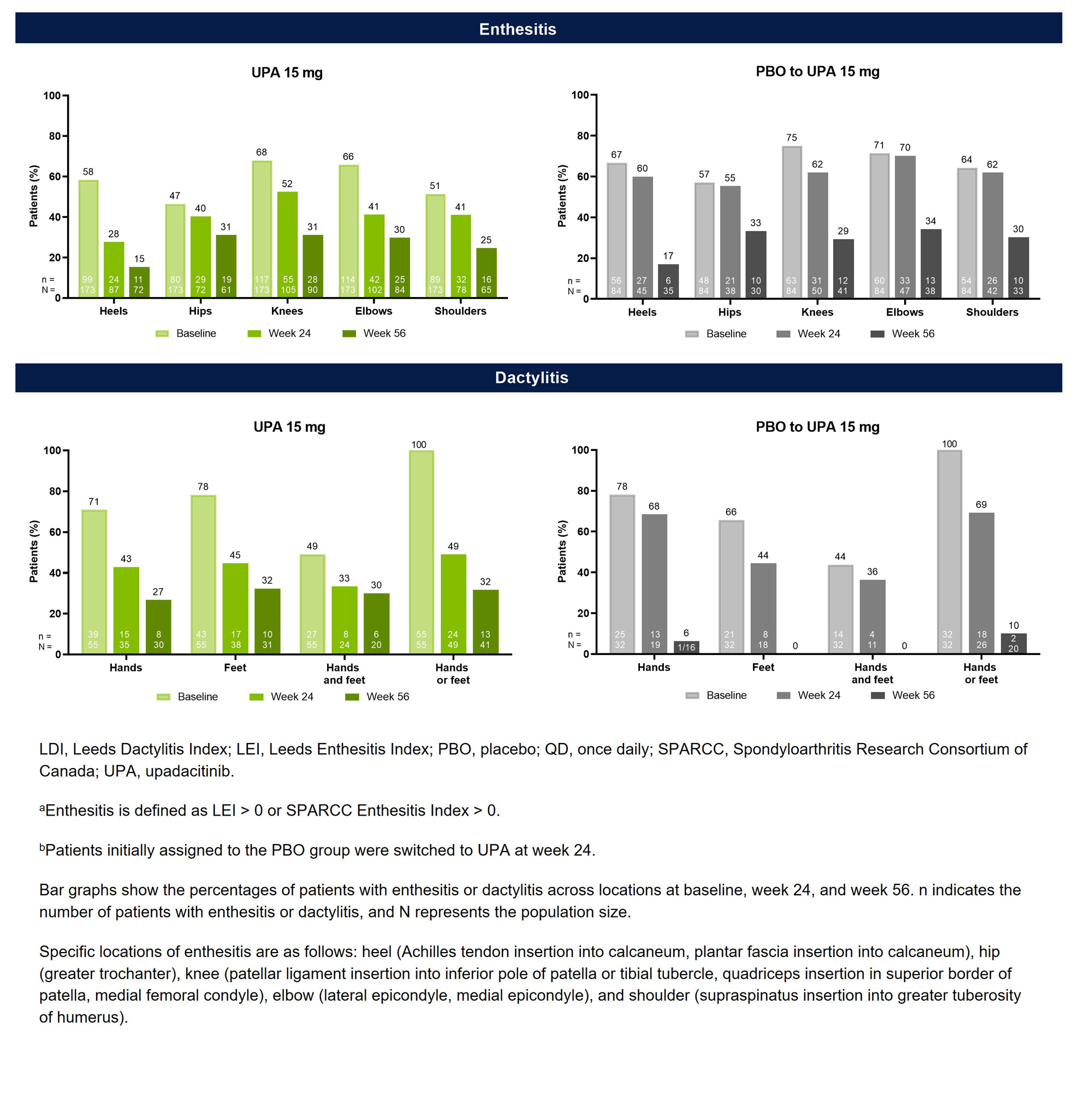
Methods: In SELECT-PsA 2, patients with an inadequate response or intolerance to ≥1 biologic DMARD (bDMARD-IR) were randomized to receive once daily UPA 15 mg or PBO.1 Patients initially randomized to PBO were switched to UPA at week 24. Baseline characteristics were evaluated for patients with enthesitis (defined as Leeds Enthesitis Index [LEI] or SPARCC Enthesitis Index >0) or dactylitis (defined as Leeds Dactylitis Index [LDI] >0). Among patients with baseline involvement, the presence of enthesitis (shoulders, elbows, hips, knees, and heels) and dactylitis (hands, feet, both hands and feet, and either hands or feet) was assessed at baseline, week 24, and week 56 in patients randomized to UPA 15 mg and in those who switched from PBO to UPA 15 mg at week 24.
Results: Of the 423 patients receiving UPA 15 mg (n=211) or PBO (n=212) in SELECT-PsA 2, 344 (81%) had baseline enthesitis or dactylitis. More patients had baseline enthesitis involvement (n=257) than dactylitis (n=87), although subgroups were non-exclusive, and patients could present with both. The mean LEI and LDI scores at baseline were ~3.1 and ~113.2 for patient subgroups with enthesitis and dactylitis, respectively, indicating a relatively high disease burden (Table 1). Across all examined locations, treatment with UPA resulted in greater decreases in the proportions of patients with enthesitis or dactylitis compared to PBO at week 24 (Figure 1). Notably, all sites of enthesitis or dactylitis involvement demonstrated improvement with UPA, ranging from a 30-percentage point reduction from baseline for heel enthesitis to a 7-percentage point reduction for hip enthesitis, as well as a 51-percentage point reduction in dactylitis of the hands or feet. The proportions of UPA-treated patients with enthesitis or dactylitis continued to decrease from week 24 to week 56. Additionally, patients who switched from PBO to UPA 15 mg at week 24 were able to achieve similar responses as the continuous UPA group by week 56.
Conclusion: UPA treatment was effective in controlling enthesitis and dactylitis at all evaluated locations in bDMARD-IR PsA patients at week 24, including locations where the disease burden may significantly impact quality of life, with continued improvements evident at week 56. However, results should be interpreted with caution given the small sample size for some subgroups, particularly dactylitis.
References
1. Mease, PJ et al. N Engl J Med 2017; 377: 1537-50.
To cite this abstract in AMA style:
Coates L, Bakewell C, Khraishi M, Chen S, Gao T, Setty A, Jones H, Ciecinski S, Mysler E. Impact of Upadacitinib on Enthesitis and Dactylitis by Location in Patients with Psoriatic Arthritis and an Inadequate Response to Biologic DMARDs from the SELECT-PsA 2 Trial [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/impact-of-upadacitinib-on-enthesitis-and-dactylitis-by-location-in-patients-with-psoriatic-arthritis-and-an-inadequate-response-to-biologic-dmards-from-the-select-psa-2-trial/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-upadacitinib-on-enthesitis-and-dactylitis-by-location-in-patients-with-psoriatic-arthritis-and-an-inadequate-response-to-biologic-dmards-from-the-select-psa-2-trial/