Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral JAK inhibitor for the treatment of RA. It was approved for RA by the US FDA in Nov 2012. TNFi cycling in non-responder patients (pts) with RA may be reinforced by payers limiting access to similarly indicated advanced therapies with different mechanisms of action, such as tofacitinib. In a retrospective analysis, adalimumab (ADA) and etanercept (ETN) were the most common prior bDMARDs for pts with RA initiating ETN and ADA, respectively, with no other advanced therapy history.1 We assessed the impact of TNFi cycling with ADA and ETN vs switching to tofacitinib.
Methods: This retrospective cohort study used the US-based IBM® MarketScan® Commercial and Medicare Supplemental insurance claims databases to identify pts with RA who newly started index medication with tofacitinib or select TNFi (ADA or ETN) between Jan 2014 and Sep 2016. Eligible pts had continuous enrolment for ≥ 12 months pre-/post-index and their only pre-index advanced therapy (bDMARD or JAK inhibitor) claim was for non-index ADA or ETN. Pts were analyzed by treatment switch: ADA to tofacitinib; ADA to ETN; ETN to tofacitinib; ETN to ADA. Outcomes assessed over 12 months post-index were: persistence without a ≥ 60-day gap in index therapy or switch; duration of therapy; effectiveness, estimated based on a validated claims-based algorithm. Changes in 12-month post-index RA-related (medical and pharmacy) costs were assessed.
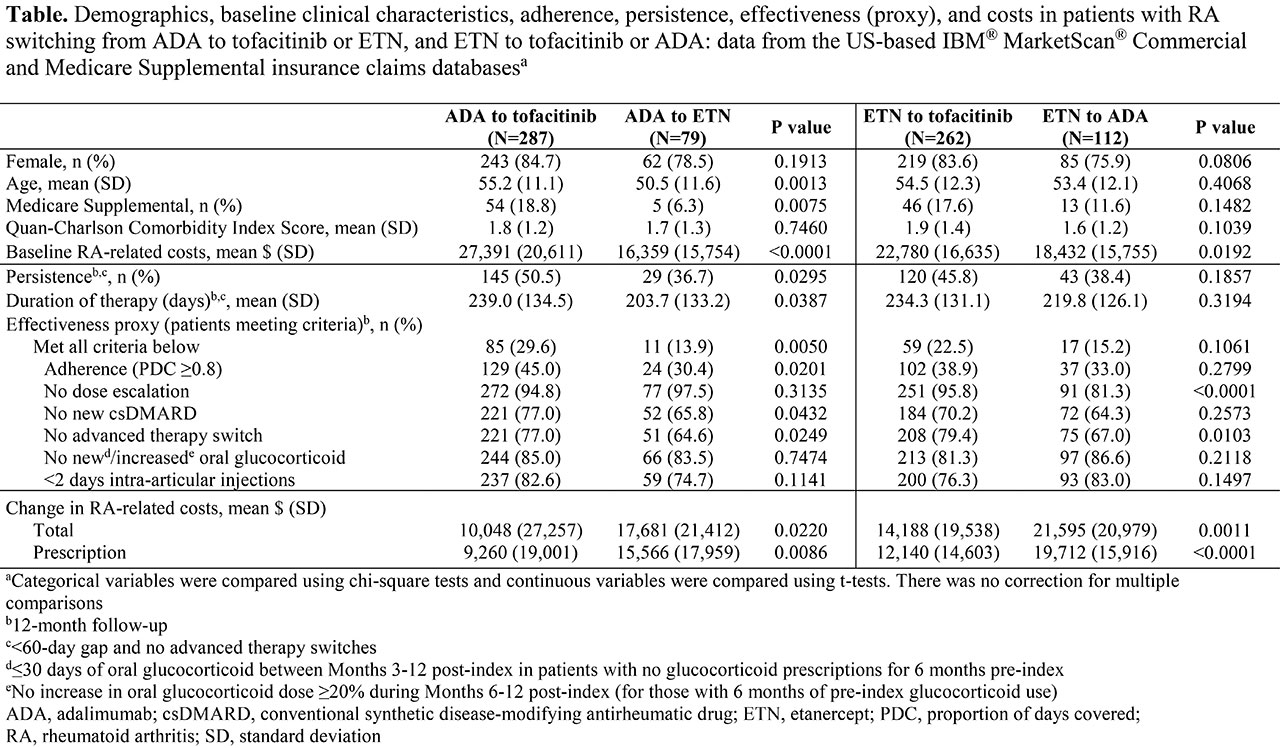
Results: Of 740 pts: 549 switched from ADA or ETN to tofacitinib; 191 cycled between ADA and ETN (Table). Pts switching to tofacitinib had significantly (p < 0.05) higher baseline (BL) RA-related costs vs pts who cycled between ADA and ETN. Pts who switched from ADA to tofacitinib were older and a greater % were Medicare Supplemental beneficiaries, vs pts who cycled from ADA to ETN. In pts who switched from ADA to tofacitinib vs those who cycled from ADA to ETN: persistence and duration of therapy were significantly higher and longer, respectively; and the % effectively treated was significantly higher, driven by better adherence, and no new csDMARDs or advanced therapy switch. A significantly lower % of pts who switched from ETN to tofacitinib had an advanced therapy switch or dose escalation thereafter vs pts who cycled from ETN to ADA. A numerically, but not significantly, higher % of pts switching from ETN to tofacitinib were persistent and effectively treated vs pts cycling from ETN to ADA. RA-related cost increases were significantly lower in pts who switched to tofacitinib vs pts who cycled between ADA and ETN, driven by significantly smaller increases in prescription costs.
Conclusion: Pts who switched from ADA or ETN to tofacitinib had higher persistence, effectiveness, and significantly lower change in RA-related costs vs pts cycling between ADA and ETN. Further adjusted analyses are planned to understand potential delays in tofacitinib use due to access barriers promoting 1st-line TNFi use. Larger sample sizes may be needed to further evaluate statistical differences for pts switching from ETN to tofacitinib vs ADA.
- Harnett J et al. J Manag Care Spec Pharm 2016; 22: 1457-1471.
Acknowledgments: Study sponsored by Pfizer Inc. Medical writing support was provided by Sarah Piggott of CMC Connect and funded by Pfizer Inc.
To cite this abstract in AMA style:
Harnett J, Smith T, Woolcott J, Gruben D, Murray C. Impact of TNF Inhibitor Cycling with Adalimumab and Etanercept vs Switching to Tofacitinib [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/impact-of-tnf-inhibitor-cycling-with-adalimumab-and-etanercept-vs-switching-to-tofacitinib/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-tnf-inhibitor-cycling-with-adalimumab-and-etanercept-vs-switching-to-tofacitinib/