Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: A previous meta-analysis1 showed that tapering of bDMARDs does not increase the risk of relapse in rheumatoid arthritis (RA) patients with remission or low disease activity (LDA). In the present meta-analysis we assessed the impact of tapering targeted therapies (bDMARDs or Jakinibs) on the risk of adverse events (AEs) of special interest in patients with RA or spondyloarthritis (SpA) in remission or (LDA).
Methods: A systematic literature analysis was carried out through May 2019 on PubMed, Embase, Cochrane and international meeting databases, selecting controlled trials comparing tapering (dose reduction or spacing) versus continuation of the initial treatment regimen, in patients with RA or SpA in remission or LDA. The meta-analysis assessed the risk difference and 95% confidence interval (95%CI) of serious infections, serious AEs, malignancy or cardiovascular AEs after tapering versus continuation of bDMARDs or Jakinibs.
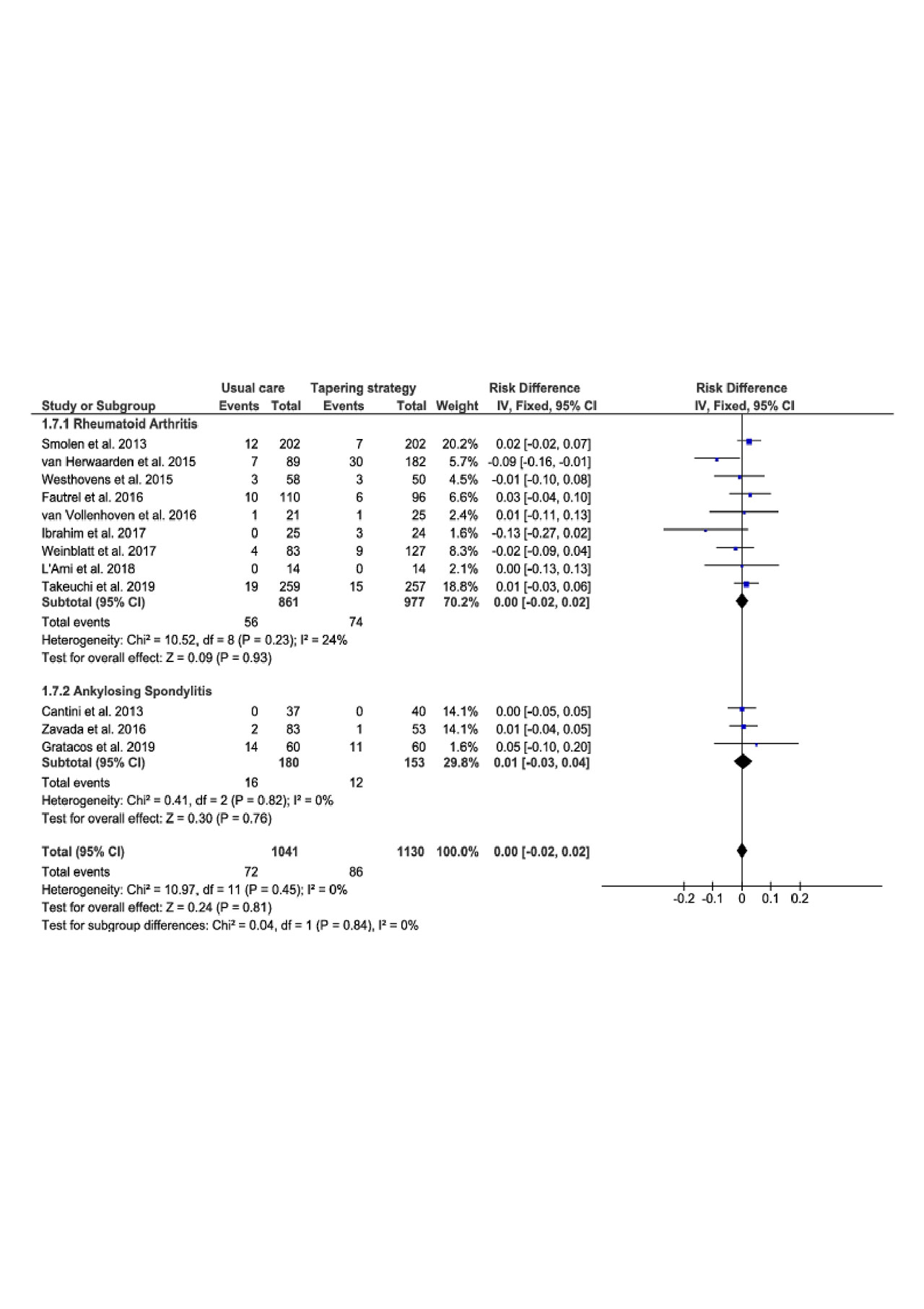
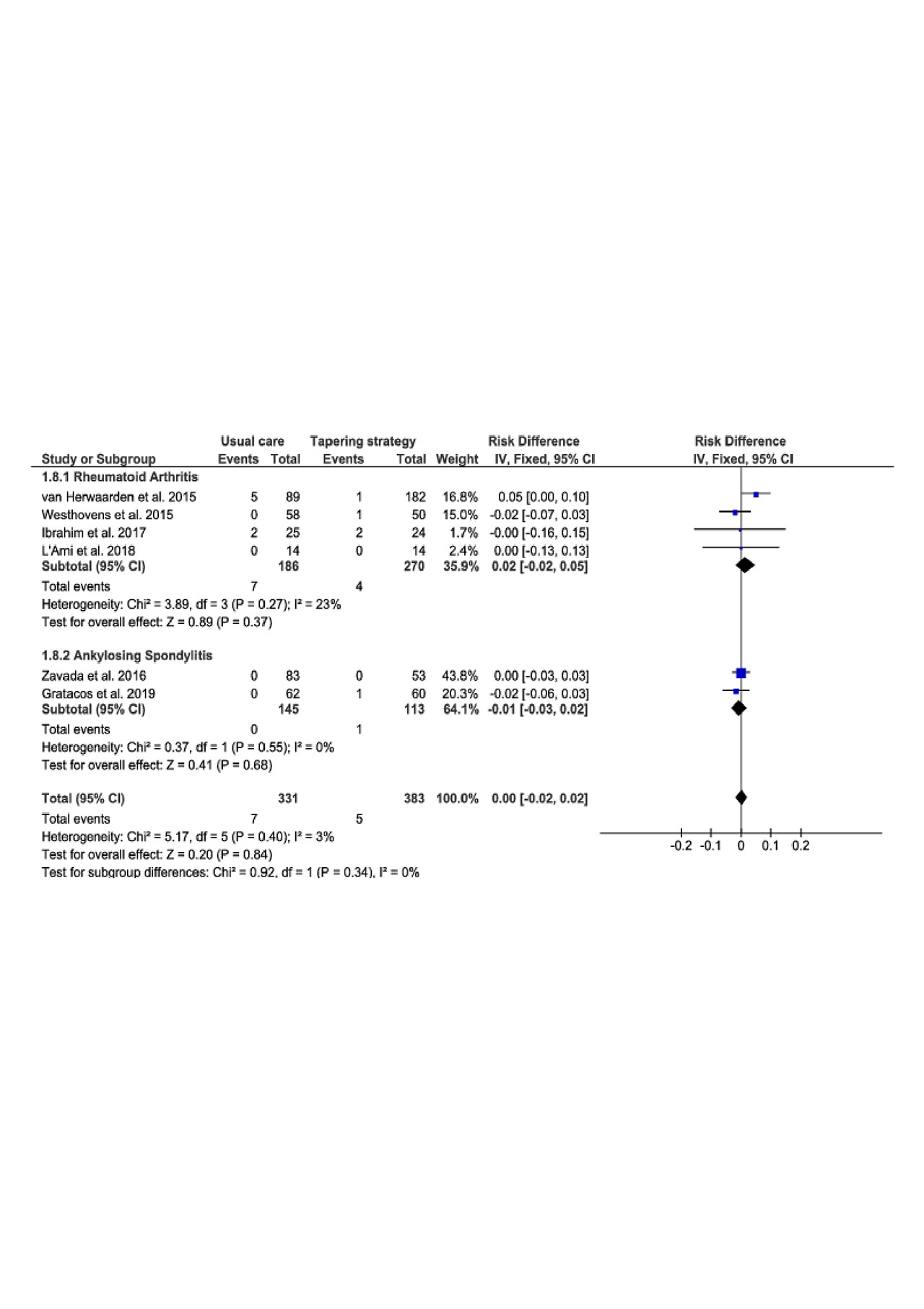
Results: 1922 records were screened in our systematic analysis of literature. 1826 records were excluded after title and abstract reading leaving 96 articles eligible. After full text assessment, 84 references were excluded. Twelve references were finally included in our systematic review and meta-analysis among which 9 trials refered to RA and 3 trials to SpA.
There were 1129 patient-year studied in the targeted therapy tapering group (TG) versus 1041 in the usual care group (UC). The study period lasted 6 months in 2 trials, 12 months in 7 trials and around 18 months in 3 trials. Mean years from diagnosis was 8 years and extended from 2.6 years to 16.6 years, the sex ratio was 67% and mean age was 51 years in both groups.
The meta-analysis comparing targeted therapy tapering versus continuation performed on twelve trials showed no decreased risk of serious infections (Risk difference (95% CI) = 0.01 (-0.00 to 0.02), P = 0.12), serious AEs (Risk difference (95% CI) = 0.00 (-0.02 to 0.02), P = 0.81), malignancy (Risk difference (95% CI) = -0.01 (-0.02 to 0.01), P = 0.33) or cardiovascular AEs (Risk difference (95% CI) = 0.00 (-0.02 to 0.02), P = 0.84). There was no difference in subgroup analysis (RA and SpA).
Conclusion: In this meta-analysis focused on controlled trials in RA and SpA patients in remission or LDA, tapering bDMARDs or Jakinibs does not lead to a decreased risk of serious infections, serious AEs, malignancy or cardiovascular AEs in comparison with continuation of the initial treatment regimen.
References
1Henaux S, et al. Ann Rheum Dis 2018;77:515-22.
To cite this abstract in AMA style:
VINSON D, MOLLET-BENHAMOU L, Degboe Y, Pham T, BARNETCHE T, Constantin A, Ruyssen-Witrand A. Impact of Tapering Targeted Therapies (bDMARDs or Jakinibs) on the Risk of Adverse Events of Special Interest in Patients with Rheumatoid Arthritis or Spondyloarthritis: A Systematic Analysis of the Literature and Meta-analysis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/impact-of-tapering-targeted-therapies-bdmards-or-jakinibs-on-the-risk-of-adverse-events-of-special-interest-in-patients-with-rheumatoid-arthritis-or-spondyloarthritis-a-systematic-analysis-of-the-l/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-tapering-targeted-therapies-bdmards-or-jakinibs-on-the-risk-of-adverse-events-of-special-interest-in-patients-with-rheumatoid-arthritis-or-spondyloarthritis-a-systematic-analysis-of-the-l/