Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Medicare Part B drug expenditures have increased due to the rising use of expensive specialty drugs like provider-administered biologics. These medications can also be billed under Part D, potentially reducing cost incurred by patients, particularly for those eligible for low-income subsidies. However, billing under Part D involves more steps and office visits, which may impact treatment compliance. Romosozumab (romo) is a monthly provider-administered biologic for women with postmenopausal osteoporosis (OP) at high risk of fracture, primarily billed under Part B. This study examined the impact of billing romo under Part D versus Part B on discontinuation among US postmenopausal women newly starting romo. We hypothesized higher discontinuation risk for those billed romo under Part D compared to those billed it under Part B.
Methods: Using fee-for-service Medicare data, women over 65 initiating romo between 10/1/2019 and 9/30/2020 were included, excluding those with metastatic cancer or Paget’s disease. Romo discontinuation was defined as a gap of over 60 days between injections. Patients billing their first romo under Part D were classified as the Part D group; others were classified as the Part B group. Follow-up ended at first discontinuation, completion of 1-year or 12-dose romo treatment, death, end of coverage, study period (12/31/2021), or first billing switch (Part B to D or vice versa), whichever came first. Propensity scores were used to account for channeling bias by using a logistic regression with covariates including demographics, fracture/OP treatment history, comorbidities, dual-eligible status, provider specialty, quarter of romo initiation, rural-urban category, out-of-pocket drug spending, and county socioeconomic status. A Cox proportional hazard model, stratified by propensity score decile, evaluated the association between billing group and romo discontinuation. Sensitivity analyses tested the robustness of this association regarding billing switches.
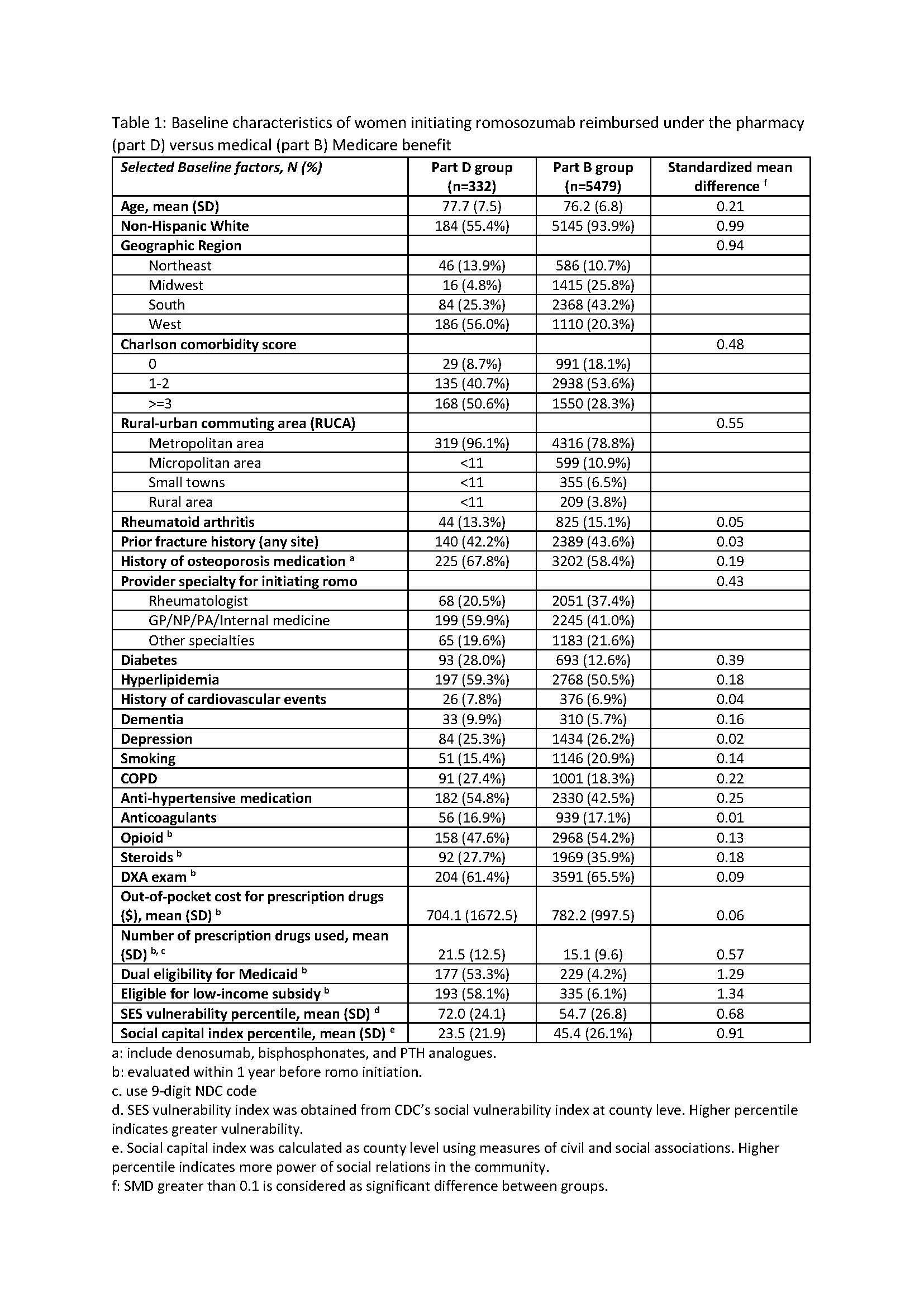
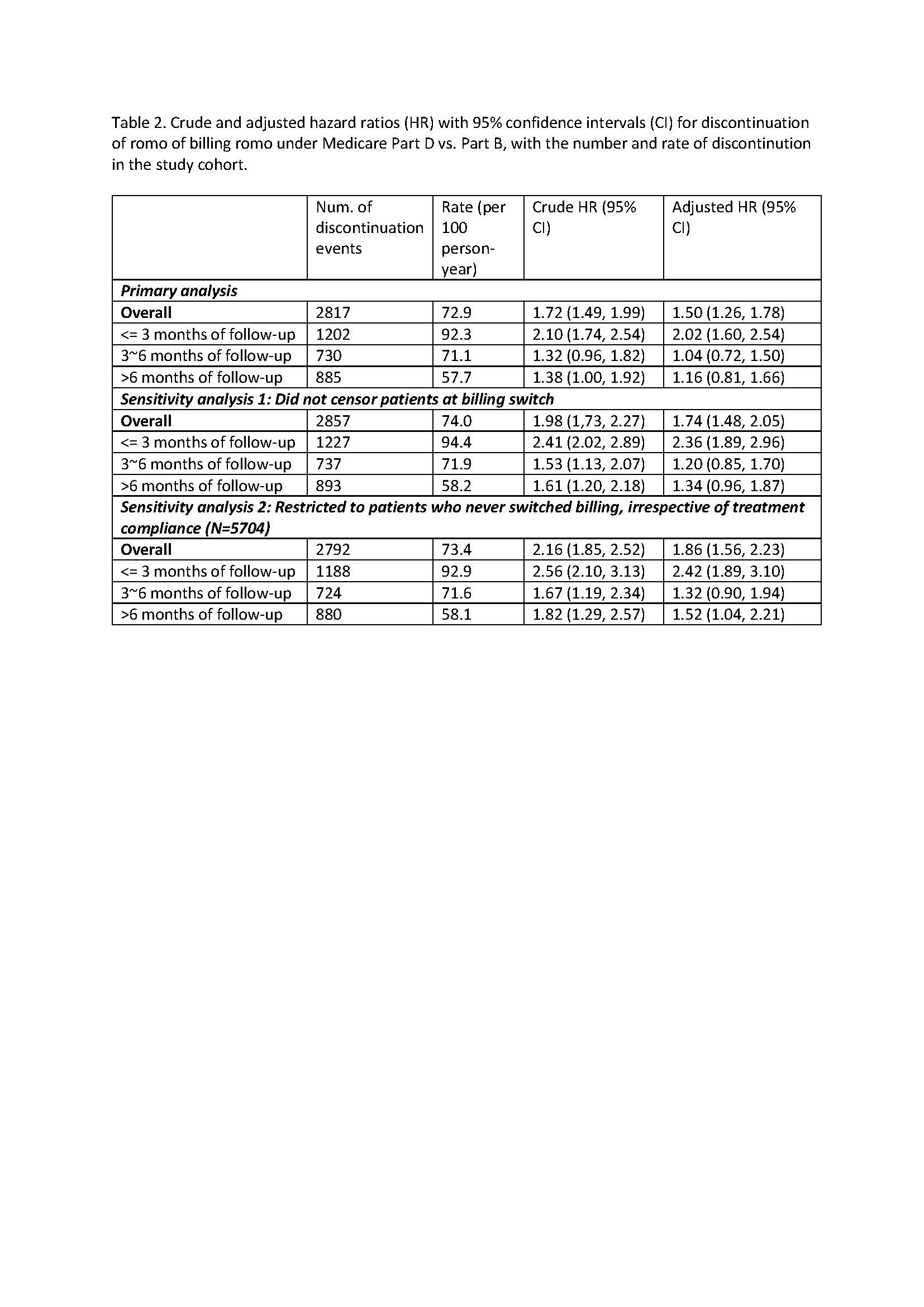
Results: Among 5811 romo users identified, 332 had their first dose billed under the part D pharmacy benefit. Baseline characteristics are shown in Table 1. Notably, patients in Part D group were older, less likely to be non-Hispanic White, were more likely to reside in counties with high SES vulnerability and low social capital, had more comorbidities, were less likely to receive romo from rheumatologists, used more prescription drugs, and were more likely to be eligible for Medicaid (dual eligible) or low-income subsidies. Results from Cox regression models including sensitivity analyses are shown in Table 2. Patients billing romo under Part D had a higher risk of discontinuation (Adjusted HR=1.50, p< 0.001) compared to those billing romo under Part B, particularly in early treatment period.
Conclusion: Medicare beneficiaries billing romosozumab under Part D faced increased discontinuation risk after adjusting for comorbidities, healthcare utilization, and SES factors, particularly in the early treatment period. These findings highlight the need for streamlined healthcare delivery processes and targeted support for timely access to medication among postmenopausal women with OP.
To cite this abstract in AMA style:
Liu Y, Zhang J, Arora T, Sen B, Saag K, Curtis J. Impact of Provider Billing vs. Pharmacy Dispensation for Romosozumab Treatment Discontinuation Among U.S. Postmenopausal Women with Osteoporosis [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impact-of-provider-billing-vs-pharmacy-dispensation-for-romosozumab-treatment-discontinuation-among-u-s-postmenopausal-women-with-osteoporosis/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-provider-billing-vs-pharmacy-dispensation-for-romosozumab-treatment-discontinuation-among-u-s-postmenopausal-women-with-osteoporosis/