Session Information
Date: Sunday, November 13, 2022
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Chronic activation of interferon (IFN) pathways is a key driver of immune dysregulation in SLE. Hydroxychloroquine (HCQ) inhibits toll-like receptors 7 and 9 in plasmacytoid dendritic cells resulting in decreased release of interferon alpha1. Studies in SLE are limited by patient heterogeneity and the superimposed complexity of combined medications they receive. This project compared activation of Type I and II interferon (IFN) pathways in SLE patients with discrete immune phenotypes who were taking hydroxychloroquine or not (HCQ vs No Rx) without corticosteroids or any other SLE treatments.
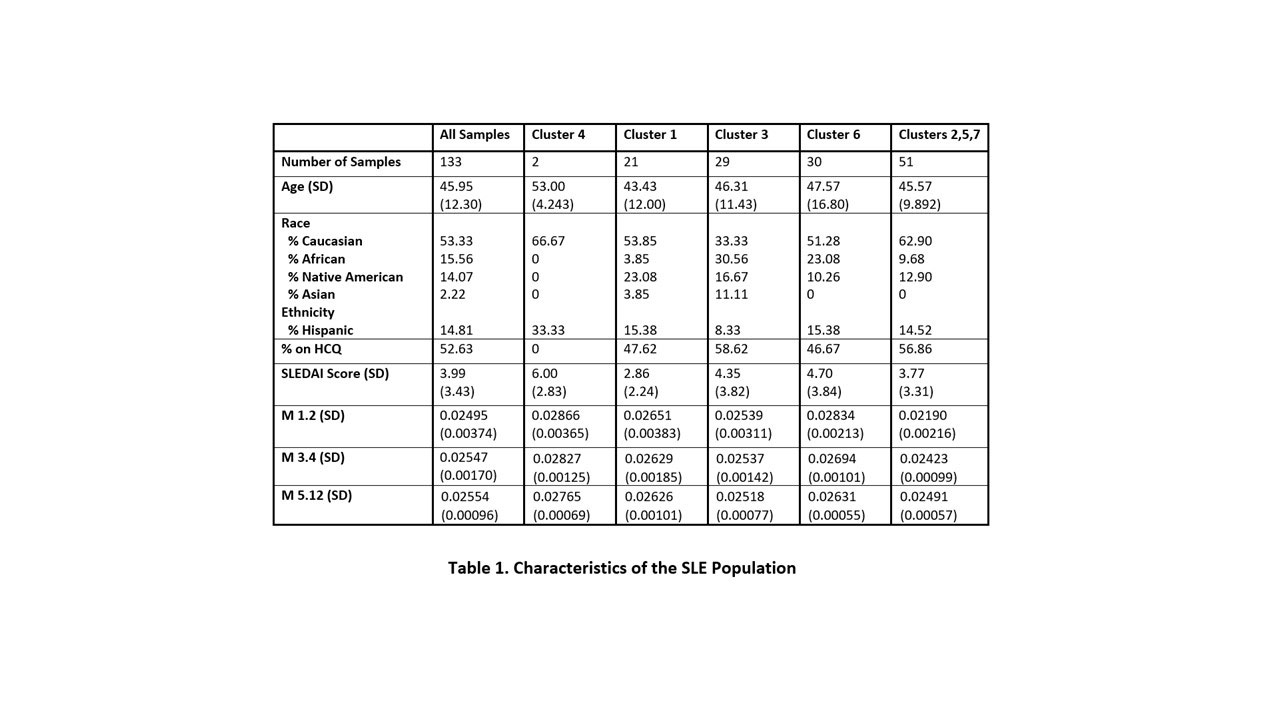
Methods: 133 samples from 85 SLE patients (70 on HCQ, 63 on No Rx) (Table 1) and 37 healthy controls were studied. Characteristic clusters of patients who shared immune activation patterns were identified by Random Forest modeling of soluble mediators and gene co-expression module scores, as previously reported2,3. Samples from four of these SLE immunologic clusters (C1, C4, C3, and C6), all with elevated expression of IFN modules (M1.2, M3.4, and M5.12) were examined for impact of HCQ on IFN pathway expression and compared to clusters with lower interferon signals. Dual samples from each of 21 patients were also evaluated when on HCQ vs on No Rx at different timepoints. Additionally, 25 samples from patients on methotrexate plus HCQ were compared to the 70 on HCQ alone.
Results: As expected, expression of IFN pathway modules were increased in the overall SLE population vs healthy controls (p< 0.001), driven by patient phenotype clusters 1,3,4 and 6. All IFN modules were decreased in SLE patients on HCQ vs. those on no Rx (M1.2 p=0.035, M3.4 p=0.009, and M5.12 p=0.005), again promulgated by changes in clusters 1,3,4 and 6 (Fig 2). In multilinear regression modeling, HCQ had independent effects on M1.2 (p=0.004), M4.3 (p < 0.001), and M5.12 (p < 0.001) after adjusting for cluster and race. Age and SLEDAI score had no confounding effects. Of the 21 patients with samples on HCQ vs No Rx, IFN scores were lower when on HCQ by Wilcoxon matched pairs signed rank test with Holm-Sidak correction (M1.2 p=0.0125, M3.4 p=0.0125, M5.12 p=0.0048). Patients in high IFN clusters taking methotrexate + HCQ had slightly lower expression of plasma cell and T cell modules compared to HCQ alone, but also demonstrated increased expression in interferon and several inflammatory pathways (Fig 3).
Conclusion: This study confirmed previous reports that hydroxychloroquine dampens interferon pathways in SLE patients, while addressing potentially confounding effects of polypharmacy and differing immune activation patterns in these patients. Limitations include inability to include more ill patients who inevitably require more complex treatment regimens, given current lack of precision medicine guidance, and not having HCQ levels to improve data interpretability. The results support the feasibility of strategies to improve patient selection for HCQ, optimize its dosing, and more rationally select combination treatments.
1. Sacre Arth Res Ther. 2012 14:R155.
2. Guthridge EClinMed. 2020 20: 100291
3. Chiche Arth Rheumatol 2014 66:1583
To cite this abstract in AMA style:
Mowery C, Smith M, Thomas K, Macwana S, deJager W, Kamp S, Arriens C, Aberle T, James J, Guthridge J, Merrill J. Impact of Hydroxychloroquine on Interferon Pathway Expression of SLE Phenotypic Subsets in the Absence of Background Medications [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/impact-of-hydroxychloroquine-on-interferon-pathway-expression-of-sle-phenotypic-subsets-in-the-absence-of-background-medications/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-hydroxychloroquine-on-interferon-pathway-expression-of-sle-phenotypic-subsets-in-the-absence-of-background-medications/