Session Information
Date: Saturday, November 16, 2024
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Glucocorticoid is a critical treatment option for systemic lupus erythematosus (SLE). However, long-term use is associated with numerous side effects and an increased risk of organ damage and mortality, necessitating early dose reduction. Although several studies have addressed the achievement rate of glucocorticoid reduction in SLE, there is limited information regarding the risk and protective factors involved. This study aims to fill that gap.
Methods: We conducted a retrospective analysis of medical records for SLE patients who received follow-up care at St. Luke’s International Hospital between April 2006 and February 2023. We included patients with new-onset SLE treated at our center, and those requiring prednisone (PSL) >20 mg/day to manage initial symptoms. We addressed the time to achieve PSL ≤7.5 mg/day and PSL ≤5 mg/day and its risk and protective factors. We used the Kaplan–Meier method and log-rank test to analyze the cumulative incidence of achieving these outcomes. A Cox proportional hazards model was employed to calculate the hazard ratio (HR) for each parameter.
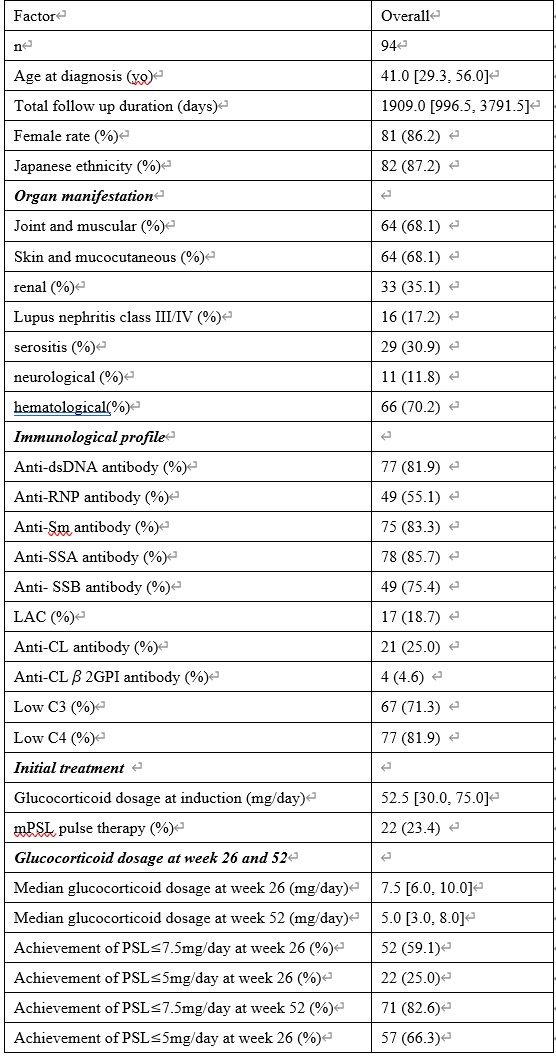
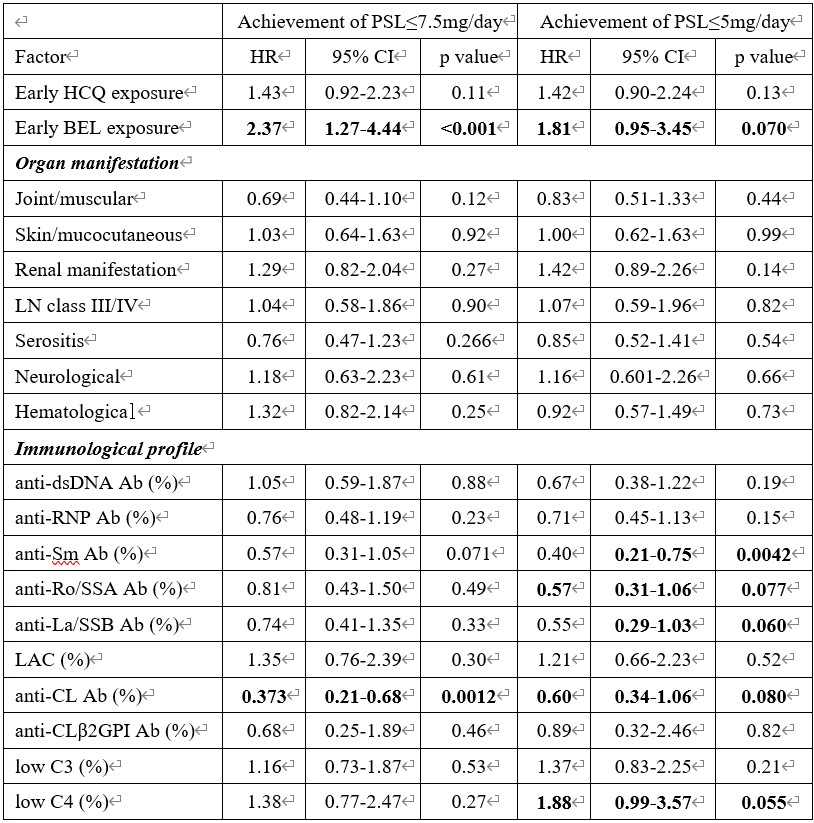
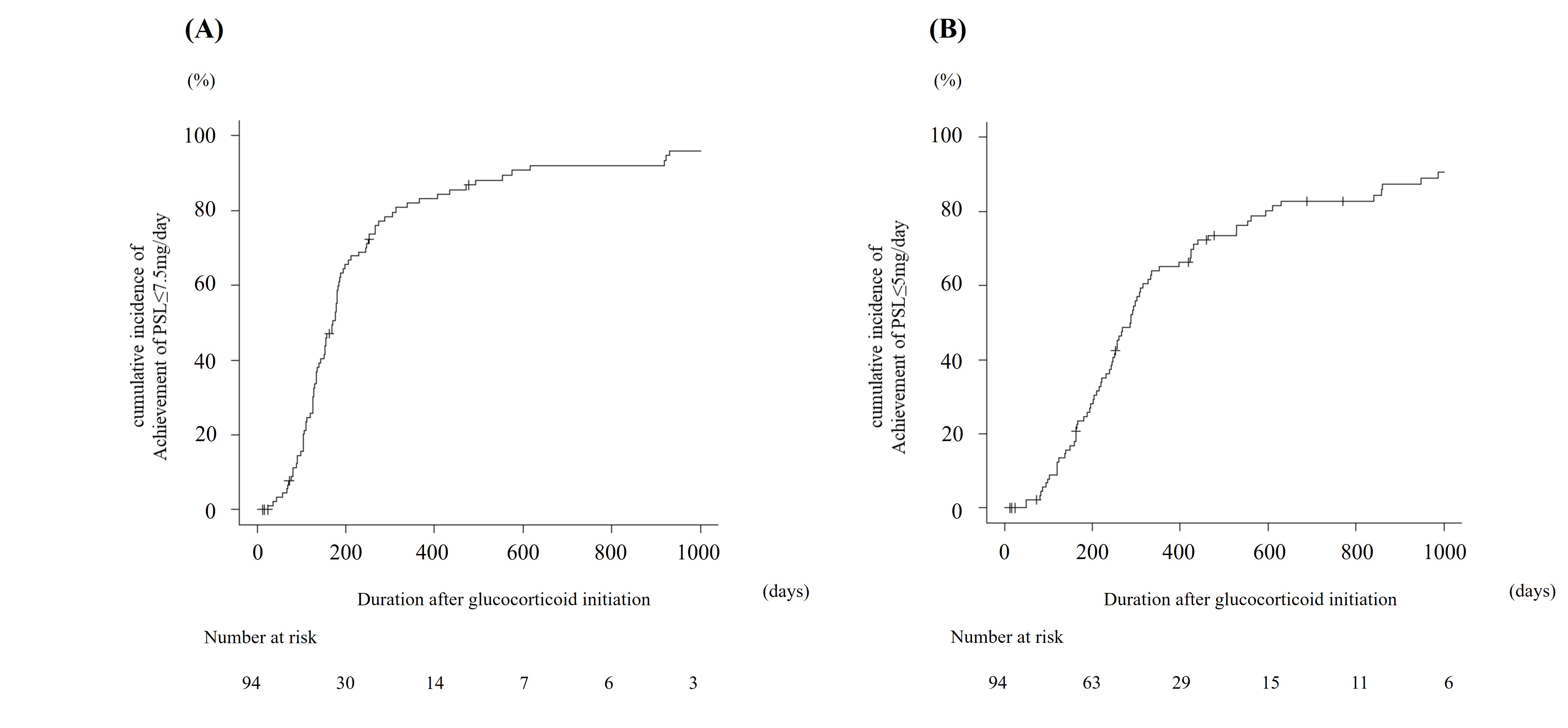
Results: Of the 510 patients diagnosed with SLE, 94 met the inclusion criteria. The median initial induction dose of glucocorticoid was 52.50 [30.00, 75.00] mg/day, which was reduced to 7.50 [6.00, 10.00], and 5.00 [3.00, 8.00] mg/day at weeks 26, and 52, respectively. By week 52, 82.6% of patients achieved PSL ≤7.5 mg/day, and 66.3% achieved PSL ≤5 mg/day. Univariate Cox proportional hazards model analysis indicated that early induction with belimumab and HCQ was associated with an increased rate of achieving PSL ≤7.5 mg/day and PSL ≤5 mg/day (early belimumab exposure: achieving PSL ≤7.5 mg/day: HR 2.37, 95% CI 1.27-4.44, p < 0.001; achieving PSL ≤5 mg/day: HR 1.81, 95% CI 0.95-3.45, p = 0.070) (early HCQ exposure: achieving PSL ≤7.5 mg/day: HR 1.43, 95% CI 0.92-2.23, p = 0.11; achieving PSL ≤5 mg/day: HR 1.42, 95% CI 0.90-2.24, p = 0.13). Positivity for anti-Sm, anti-Ro/SSA, and anti-La/SSB antibodies was associated with a decreased likelihood of achieving PSL ≤5 mg/day (anti-Sm antibody: HR 0.40, 95% CI 0.21-0.75, p = 0.0042; anti-Ro/SSA antibody: HR 0.57, 95% CI 0.31-1.06, p = 0.077; anti-La/SSB antibody: HR 0.55, 95% CI 0.29-1.03, p = 0.060). No organ manifestations, including lupus nephritis and neuropsychiatric SLE, were related to the rate of achieving PSL ≤7.5 mg/day or PSL ≤5 mg/day.
Conclusion: The use of HCQ and belimumab was associated with an increased likelihood of achieving glucocorticoid reduction in patients with new-onset SLE.
To cite this abstract in AMA style:
Nakai T, Fukui S, Ikeda Y, Tamaki H, Kishimoto M, Okada M. Impact of Hydroxychloroquine and Belimumab on Early Glucocorticoid Reduction in New-Onset Systemic Lupus Erythematosus: A Retrospective Analysis of Risk and Protective Factors [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impact-of-hydroxychloroquine-and-belimumab-on-early-glucocorticoid-reduction-in-new-onset-systemic-lupus-erythematosus-a-retrospective-analysis-of-risk-and-protective-factors/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-hydroxychloroquine-and-belimumab-on-early-glucocorticoid-reduction-in-new-onset-systemic-lupus-erythematosus-a-retrospective-analysis-of-risk-and-protective-factors/