Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
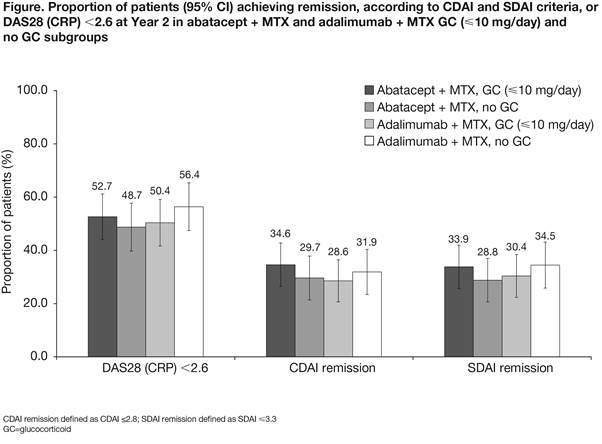
Conclusion: The use of low-dose GC with abatacept or adalimumab in RA patients with inadequate response to MTX has no impact on short-term and medium-term outcomes. This finding could be because patients who entered this trial had active disease despite background GC use and they responded to the addition of an active medication.
To cite this abstract in AMA style:
Degboé Y, Schiff M, Weinblatt M, Fleischmann R, Ahmad H, Constantin A. Impact of Glucocorticoid Therapy on the Efficacy of SC Abatacept or Adalimumab in RA Patients with Inadequate Response to MTX: A Post Hoc Analysis of Data from a Head-to-Head Trial [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/impact-of-glucocorticoid-therapy-on-the-efficacy-of-sc-abatacept-or-adalimumab-in-ra-patients-with-inadequate-response-to-mtx-a-post-hoc-analysis-of-data-from-a-head-to-head-trial/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-glucocorticoid-therapy-on-the-efficacy-of-sc-abatacept-or-adalimumab-in-ra-patients-with-inadequate-response-to-mtx-a-post-hoc-analysis-of-data-from-a-head-to-head-trial/