Session Information
Date: Sunday, November 10, 2019
Title: 3S078: RA – Diagnosis, Manifestations, & Outcomes I: Pulmonary & Other Comorbidities (839–844)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Glucocorticoids (GCs) directly impact bone metabolism via increased bone resorption and inhibited bone formation1; hence, systemic fracture risk increases with daily and cumulative GC doses.2 However, many patients with established rheumatoid arthritis (RA) receive long-term treatment with GCs to suppress inflammation, which confers some benefit in delaying bone erosion.3 This exploratory analysis of the SEMIRA (Steroid EliMination In RA) study4 compared changes in markers of bone and cartilage metabolism in RA with low disease activity (LDA) or remission on tocilizumab (TCZ) + GC ± conventional synthetic disease-modifying antirheumatic drugs (csDMARDs) who received either slow GC tapering (GC-taper) or continuation (5 mg; GC-5mg).
Methods: Patients (diagnosed per revised 1987 ACR criteria) had at least stable LDA (DAS28-ESR ≤3.2) for ≥4 weeks, were receiving a stable prednisone regimen (5 mg/day) + TCZ ± csDMARDs for ≥4 weeks, and received TCZ + GCs (prednisone equivalent 5-15 mg/day) for ≥6 months before randomization. Patients were randomly assigned to GC-5mg (n=128) or GC-taper (n=131; starting at 4 mg/day with 1-mg reduction every 4 weeks to 0 mg/day at weeks 16-24) for 24 weeks, during which TCZ ± csDMARDs remained stable. Change between baseline (BL) and week 24 in serum levels of markers of bone and cartilage metabolism—including N-terminal propeptide of type 1 collagen (P1NP), C-terminal telopeptide of type 1 collagen (CTX1), alkaline phosphatase (ALP), matrix metalloproteinase 3 (MMP3), calcium, phosphorus, and albumin—were exploratory outcomes. Within-group and between-group changes from baseline were evaluated by Wilcoxon paired rank test and Wilcoxon signed rank test, respectively.
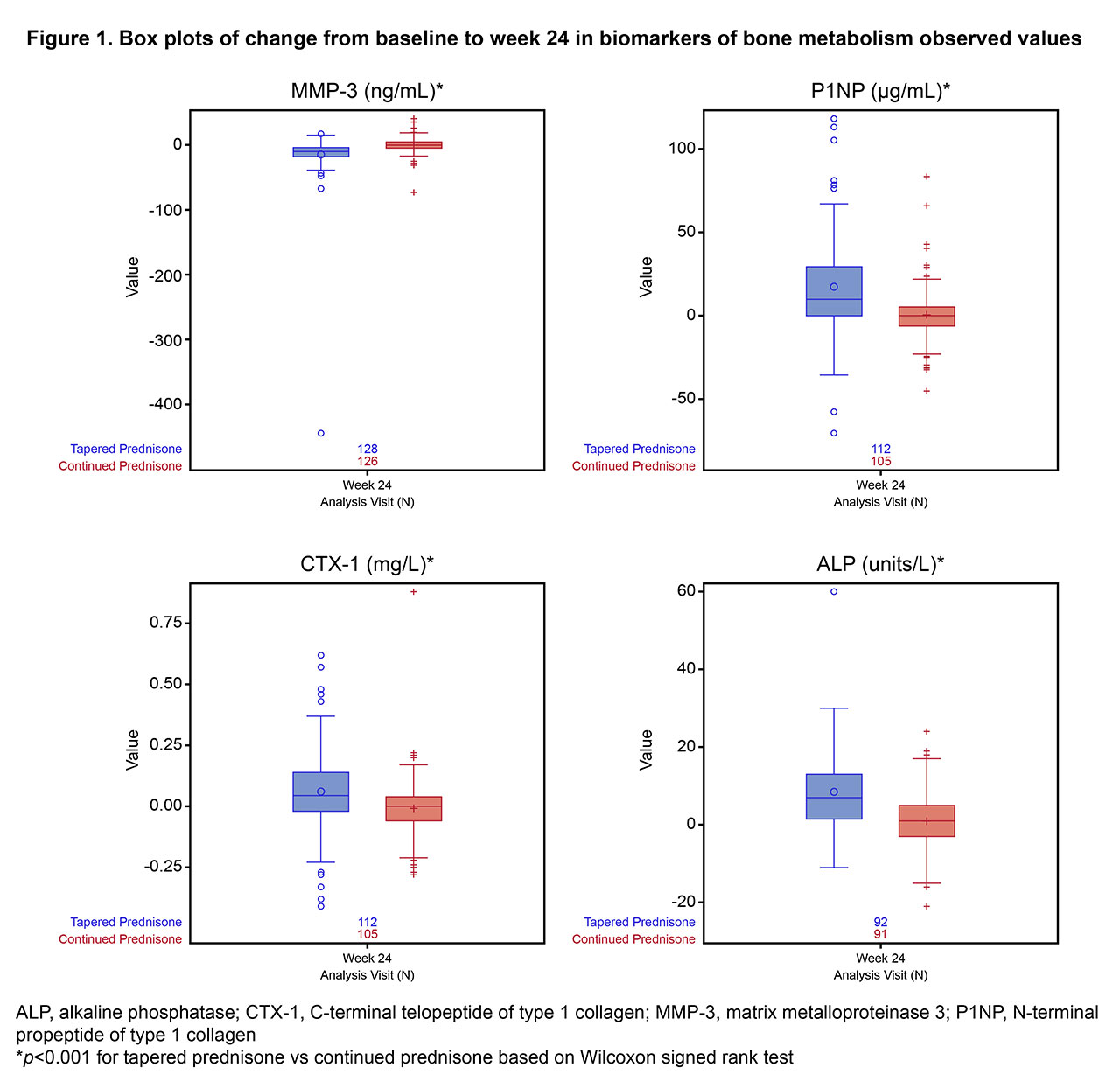
Results: Figure 1 shows changes from BL to week 24 in bone and cartilage biomarkers. MMP3 (marker of cartilage degradation) was reduced at week 24 in the GC-taper group compared with GC-5mg. Change from BL at week 24 in P1NP (bone formation) and CTX1 (bone resorption) favored the GC-taper arm, with P1NP increasing relatively more than CTX1 (44% and 24%, respectively, compared with GC-5mg), indicating net anabolism. The increase in ALP from BL to week 24 was greater for the GC-taper arm than for the GC-5mg arm, suggesting neomineralization. There was no difference in the change from BL to week 24 in calcium, phosphorus, or albumin (not shown).
Conclusion: The findings of this biochemical marker analysis suggest that withdrawal from GC after achievement of LDA or remission with TCZ results in increased bone remodeling, with a trend toward an anabolic window and reduced cartilage degradation. Given that it could take a year to recover from increased fracture risk after cessation of GC therapy,5 our results offer further insight on the reversible risk of systemic harm to noninflamed bone versus benefits for inflamed joints in the context of LDA or remission.
References: 1. Canalis E, Delany AM. Ann N Y Acad Sci 2002;966:73. 2. Van Staa TP et al. Rheumatology 2000;39:1383. 3. Kavanaugh A, Wells AF. Rheumatology 2014;53:1742. 4. Burmester GR et al. Arthritis Rheumatol 2018;70(suppl 10). 5. Vestergaard P et al. Calcif Tissue Int 2008;82:249.
To cite this abstract in AMA style:
Buttgereit F, Burmester G, Nebesky J, Devenport J, Donath M, John M. Impact of Glucocorticoid Tapering on Markers of Bone Metabolism in Patients with Rheumatoid Arthritis Who Achieved Low Disease Activity or Remission on Tocilizumab: Exploratory Analysis from a Randomized Controlled Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/impact-of-glucocorticoid-tapering-on-markers-of-bone-metabolism-in-patients-with-rheumatoid-arthritis-who-achieved-low-disease-activity-or-remission-on-tocilizumab-exploratory-analysis-from-a-randomi/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-glucocorticoid-tapering-on-markers-of-bone-metabolism-in-patients-with-rheumatoid-arthritis-who-achieved-low-disease-activity-or-remission-on-tocilizumab-exploratory-analysis-from-a-randomi/