Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Glucocorticoids (GCs) are potent anti-inflammatory drugs that are commonly prescribed, particularly for rheumatic and immunologic conditions. Hyperglycemia is an important adverse effect and occurs in approximately a third of people who receive high doses of systemic GCs. Some clinical and demographic factors are known to be associated with GC-induced hyperglycemia; however, genetic factors may also contribute. We hypothesized that genetic predisposition for type 2 diabetes (T2D) plays a role in the variably high glucose levels observed after GC administration.
Methods: We selected a cohort of individuals in the Vanderbilt University Medical Center biobank who had received a trigger dose of ≥40 mg/d prednisone-equivalents at age 18 years or older as part of clinical care. Only individuals of European ancestry (defined by genetic principal components (PCs) and HapMap reference) were selected. Eligible individuals were required to have genome-wide genotyping data available and glucose measurements before and after GC administration. The median glucose level before the trigger GC dose was calculated in all individuals, and the highest glucose level recorded within 30 days after the trigger dose was extracted and natural log transformed. Genetic predisposition for T2D was estimated using a polygenic risk score (PRS) that was built with summary genetic information from the largest genetic study of T2D in European populations and calculated in the cohort. To define the relationship between the standardized PRS for T2D and GC-induced hyperglycemia, we performed linear regression adjusting for sex, age, GC dose, median baseline glucose, and 5 PCs. P≤0.05 was considered significant. Continuous variables are shown as median [IQR] and counts as percentage. In a sensitivity analysis, we excluded individuals who had a diagnosis of diabetes before the trigger dose.
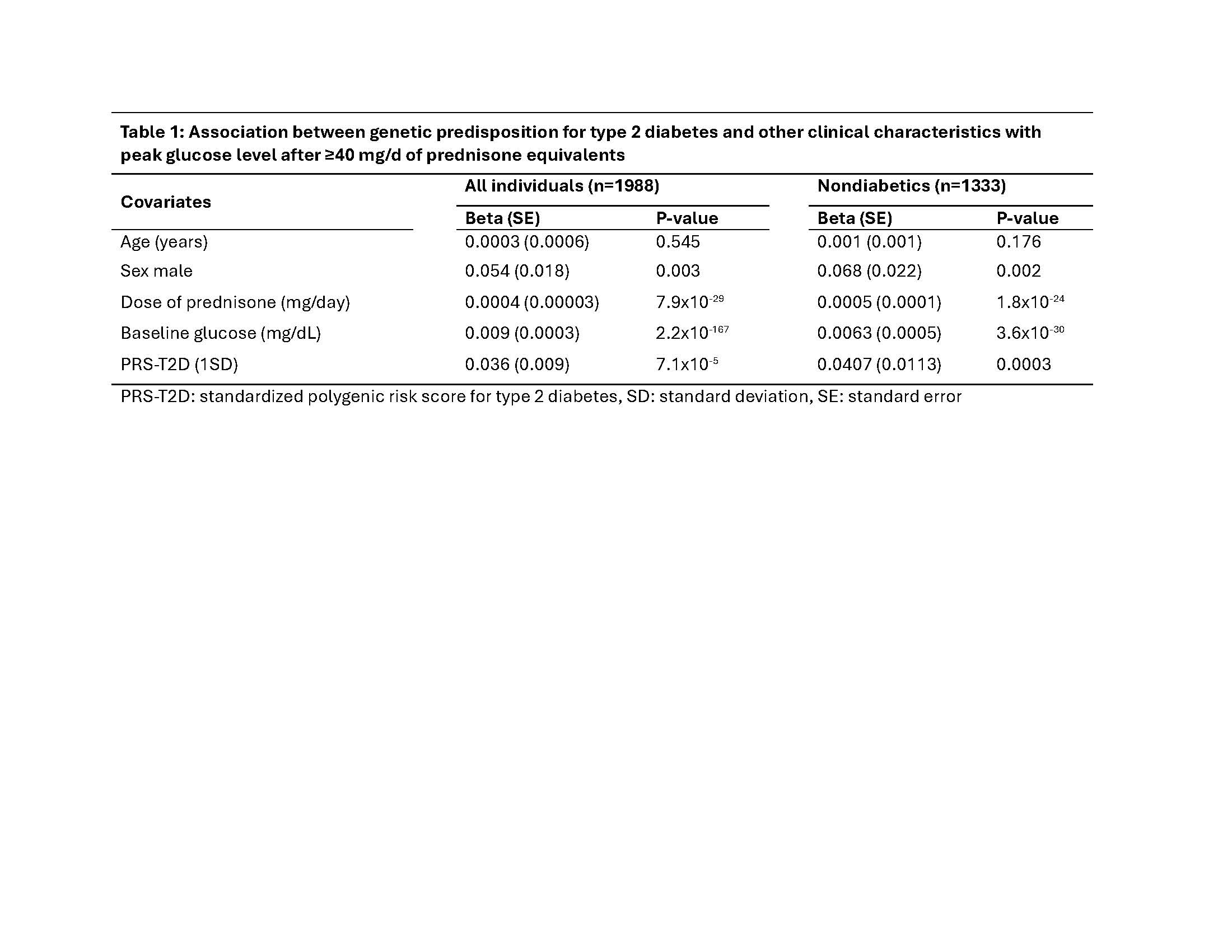
Results: There were a total of 1988 eligible individuals; of these, 12.3 percent were male, the median age and trigger GC dose were 58 [45, 66] years and 75 [40.0, 200.0] mg/d, respectively. Blood glucose increased from a baseline of 107.0 [95.9, 134.1] mg/dL to 189.5 [132.0, 283.0] mg/dL after GC. Peak glucose level within 30 days after GC administration was significantly associated with sex (P=0.003), dose of GC (P=7.9×10-29), baseline glucose (P=2.2×10-167) and with the PRS for T2D (P=7.1×10-5) (Table 1). After excluding individuals with diabetes (n=655), the association with the PRS for T2D was attenuated but remained significant (P=0.0003, Table 1)
Conclusion: Genetic predisposition for T2D plays an important role in GC-induced hyperglycemia, even in individuals without known diabetes.
To cite this abstract in AMA style:
Ruan X, Williams M, Karakoc G, McNeer E, Choi L, Mosley J, Stein C, Kawai V. Impact of Genetic Predisposition for Type 2 Diabetes in Plasma Glucose Levels After the Administration of a High Dose of Systemic Glucocorticoids [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/impact-of-genetic-predisposition-for-type-2-diabetes-in-plasma-glucose-levels-after-the-administration-of-a-high-dose-of-systemic-glucocorticoids/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-genetic-predisposition-for-type-2-diabetes-in-plasma-glucose-levels-after-the-administration-of-a-high-dose-of-systemic-glucocorticoids/