Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Changes in formulary tiers can potentially have unintended impact on outcomes. Moderate-to-severe rheumatoid arthritis (RA) is often treated with Tumor Necrosis Factor inhibitors (TNFi). A large payer implemented a formulary change on January 1, 2015 that switched the tiering and modified the copayment for two TNFi’s: etanercept (ETN) and adalimumab (ADA). The purpose of this study was to assess the effect of formulary changes on treatment patterns and healthcare utilization (HCU) costs among RA patients (pts) on TNFi, specifically ETN compared to other TNFi medications.
Methods: This claims-based, retrospective cohort study utilized Optum Clinformatics Data Mart from UnitedHealth Group. The calendar year 2015 was evaluated. Jan 1, 2015 served as the index-date; the 6 months preceding Jan 1, 2015 served as the pre-index period (baseline); and Jan 1 to Dec 31, 2015 served as the follow-up period. The cohort included pts ages ≥18, with a confirmed RA diagnosis prior to Jan 1, 2015; continuous enrollment from beginning of baseline through end of follow-up; and ≥1 TNFi claim(s) during baseline. The most proximal TNFi to the end of baseline served as the index TNFi. Pts were categorized as ETN or Other TNFi and assessed during follow-up for the first occurrence of a treatment outcome: continuer (no changes or gap ≤59 days); early-gap-restart (EGR) (≥60 but ≤180 day gap then restart index TNFi); early-switch (ES) (prescribed a different TNFi than index ≤180 days); discontinuers (no refills; restart; or switch during entire length of follow-up). HCU and mean costs were measured during follow-up.
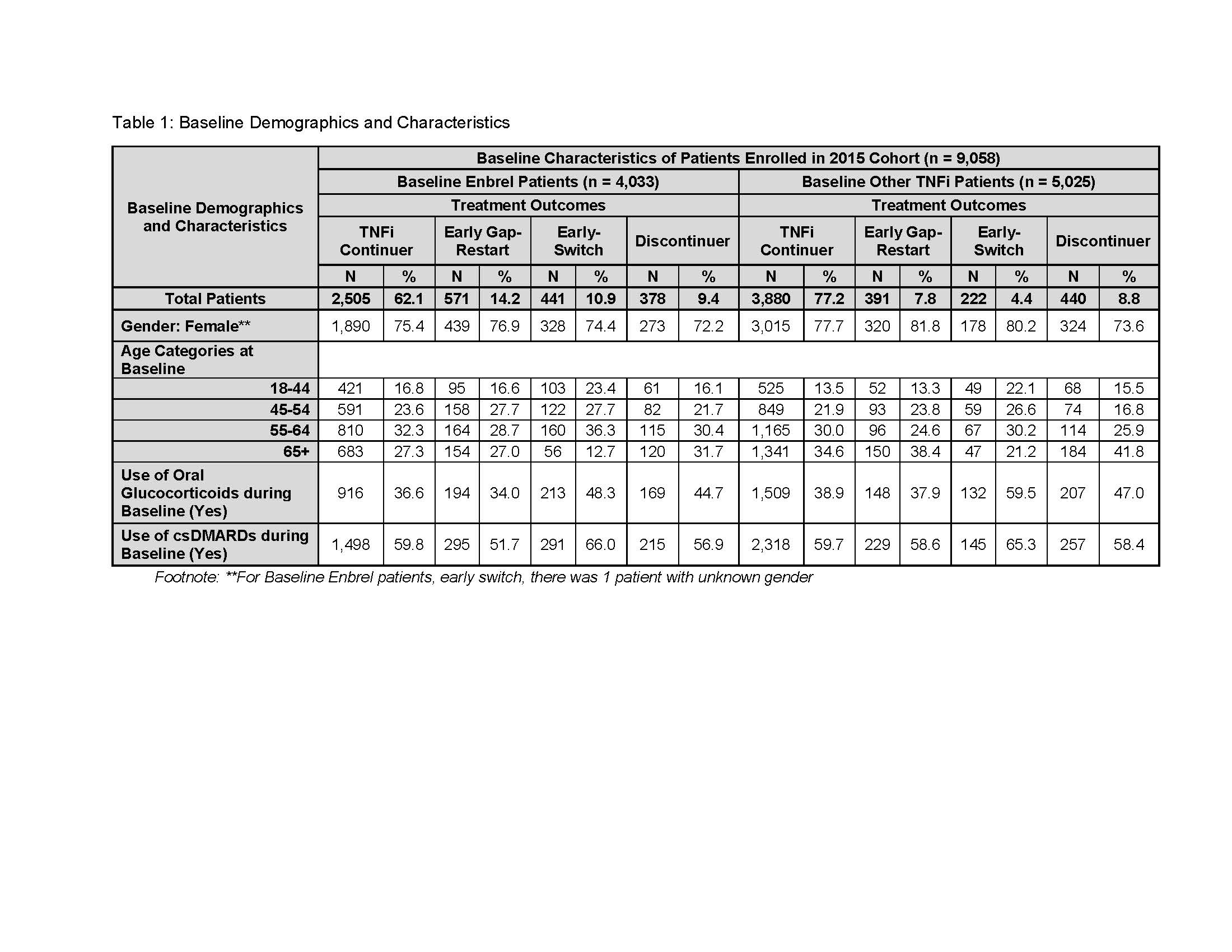
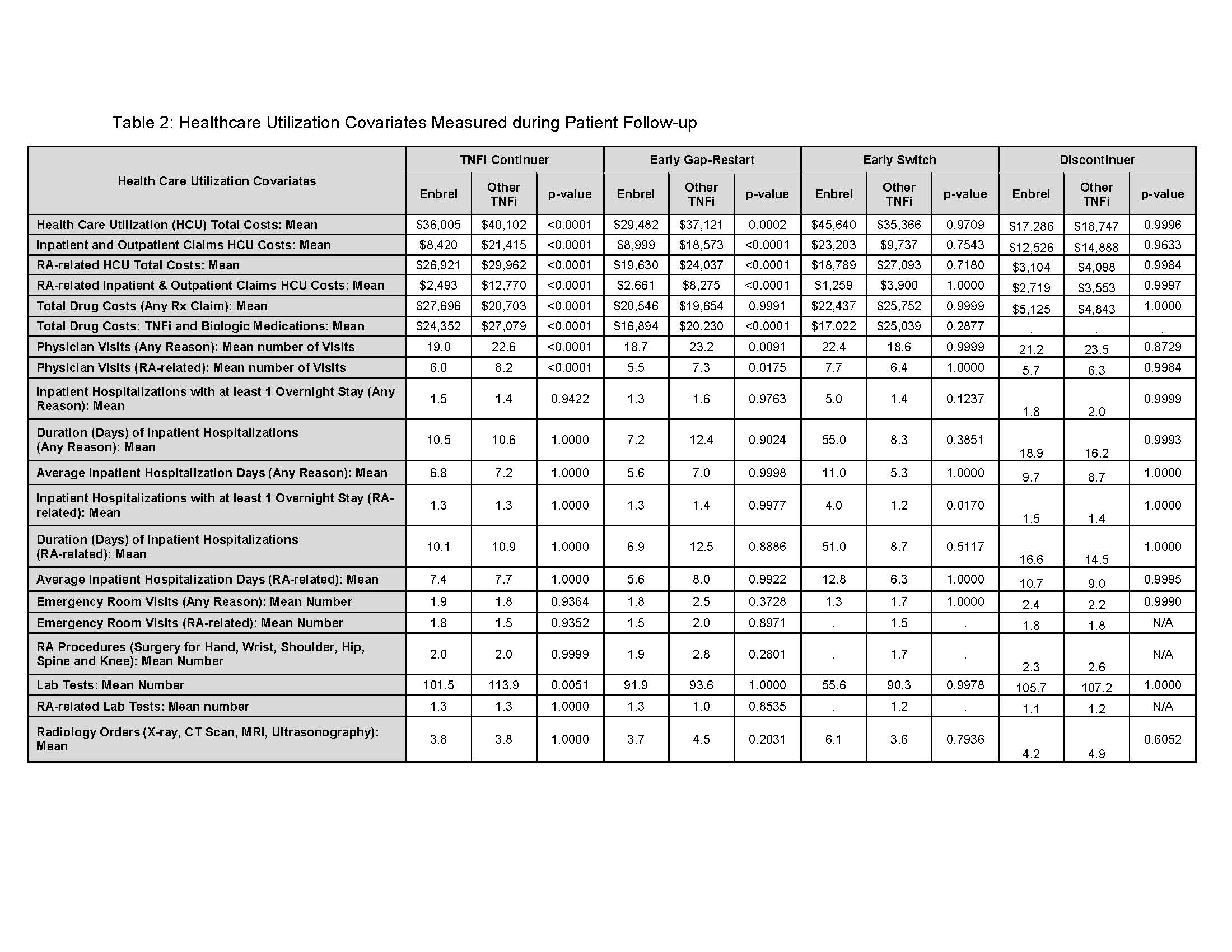
Results: In 2015, n=9,058 pts met the inclusion criteria. At baseline, ETN accounted for 44.5% (n = 4,033) of pts, of which, 62.1% continued; 14.2% EGR;10.9% ES; 9.4% discontinued, and 3.4% had a gap-restart or switch occurring ≥ 180 days. Among Other-TNFi pts (55.5%) at baseline, 77.2% continued; 7.8% EGR; 4.4% ES; 8.8% discontinued; and 1.8% had a gap-restart or switch occurring ≥ 180 days (T.1). The total mean HCU costs were lower for ETN continuers compared to Other-TNFi continuers ($36,005, $40,102; p < .0001), and for ETN EGR pts compared to Other-TNFi EGR pts ($29,482, $37,121; p = .0002) (T.2). ES ETN pts were costlier than ETN continuers (T.2). Medical costs (inpatient/outpatient) of continuers and EGR were significantly lower for ETN compared to similar pts on Other-TNFi. While total mean drug costs based on pharmacy claims for any medication were higher for ETN continuers compared to Other-TNFi continuers, and for ETN ES compared to Other-TNFi ES pts, when evaluating specifically mean costs of TNFi and biologic (pharmacy or physician-administered) they were lower for ETN continuers, EGR, and ES compared to Other-TNFi pts with the same treatment outcomes (T.2).
Conclusion: In this plan, a formulary policy change did not result in healthcare cost savings during the first year of implementation. Continuing and EGR pts using Other-TNFi incurred higher costs than similar ETN pts. Pts switching away from ETN cost more than pts switching away from Other TNFi medications. Overall, ETN patients who experienced a formulary change resulted in higher costs.
To cite this abstract in AMA style:
Schenfeld J, Stryker S, Oko-osi H, Stolshek B. Impact of Formulary Change on TNFi Treatment Patterns and Healthcare Utilization Costs in RA Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/impact-of-formulary-change-on-tnfi-treatment-patterns-and-healthcare-utilization-costs-in-ra-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-formulary-change-on-tnfi-treatment-patterns-and-healthcare-utilization-costs-in-ra-patients/