Session Information
Date: Saturday, November 7, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Secukinumab (SEC) has provided efficacy in clinical trials in patients with psoriatic arthritis (PsA). In PsA patients, a gain in response has been suggested by dose escalation from 150 to 300 mg in the open phase of the FUTURE study1. We analyze the usefulness of dose escalation of SEC from 150 to 300 in patients with non-responding PsA to 150 mg in real-world setting.
Methods: Unicentric observational, longitudinal, retrospective study conducted in a tertiary hospital between January 2016 and December 2019. Patients with PsA (CASPAR criteria) receiving at least one dose of SEC were included. Medical records were reviewed to collect demographic and clinical data related to PsA (including activity assessment and treatment).
Descriptive statistics and a comparative analysis of the efficacy of SEC by the Student t test in the different dose groups and by the ANOVA test to compare the response between the three dose groups were performed.
Results: 98 PsA patients treated with SEC, of which 69 (70%) female, were included. Mean age was 54 y.o (SD12) and average duration of the disease was 9 (SD 7) years. Three groups were performed according to the dose received, SEC150, SEC300 and SEC150-300 (non-responders after SEC150 onset increasing to 300 mg). Characteristics are detailed on Table 1. The SEC150 group includes 58 (59%) patients, SEC300 12 (12%) and SEC150-300 28 (29%) patients.
In the SEC150-300 group, 54% of the patients maintains SEC after responding to the dose increase. The average time of dose increase to 300 mg was 9 (SD6) months in this group.
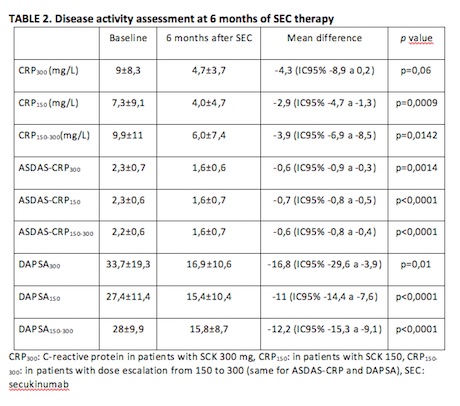
At 6 months of SEC therapy, a significant decrease in CRP, ASDAS-CRP and DAPSA values was observed in the three treatment groups (Table 2). However, when comparing the difference of means obtained during follow-up (ΔCRP, ΔASDAS-CRP and ΔDAPSA) between the 3 dose groups, no significant differences were found (p=0.76 for CRP, p=0.86 for ASDAS-CRP and p=0.35 for DAPSA).
Conclusion: There are no significant differences in the response evaluated by CRP, ASDAS-CRP and DAPSA between the dose of 150 and 300 mg of SEC. However, both doses of treatment provided efficacy in clinical practice with significant reduction of activity parameters.
In patients with non-responding PsA to SEC150 mg and prior failure to TNFi, increasing the dose to 300 mg could be an effective option.
To cite this abstract in AMA style:
Martin-Lopez M, Joven B, Pablos J. Impact of Dose Escalation of Secukinumab in Patients with Psoriatic Arthritis in Real-World Setting [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/impact-of-dose-escalation-of-secukinumab-in-patients-with-psoriatic-arthritis-in-real-world-setting/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-dose-escalation-of-secukinumab-in-patients-with-psoriatic-arthritis-in-real-world-setting/