Session Information
Date: Sunday, November 8, 2015
Title: Systemic Lupus Erythematosus - Clinical Aspects and Treatment Poster Session I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Belimumab is licensed as
add-on therapy to standard lupus care (SoC) in patients with active SLE.
Physicians have inquired about the efficacy and safety of belimumab 10 mg/kg
used in combination with various concomitant SLE medications.
Methods: We conducted a post hoc analysis
(Study 201224) of the efficacy and safety of intravenous belimumab 10 mg/kg
plus SoC versus placebo (SoC) in four baseline concomitant medication groups,
from the BLISS-52 and BLISS-76 combined database: steroids only, anti-malarials
(AM) only, steroids + anti-malarials (steroids + AM), and steroids +
anti-malarials + immunosuppressants (steroids + AM + IS). The primary endpoint
was the SLE Responder Index (SRI) at Week 52. Safety was examined using adverse
events (AEs).
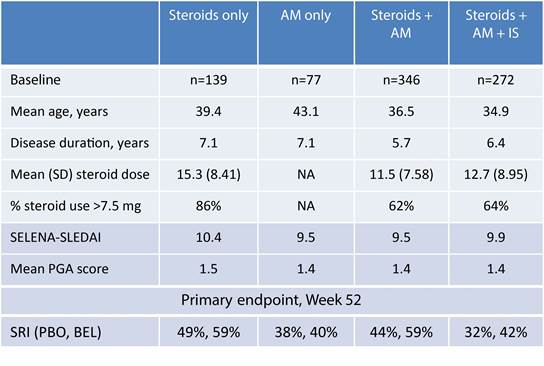
Results: The size of the baseline concomitant
medication groups varied (below). Steroid + AM was the largest concomitant
medication group and had the shortest disease duration. Patients receiving
multiple concomitant medications were younger; disease activity appeared
similar across groups. Steroid doses over 7.5 mg/day were common.
AM,
anti-malarials; BEL, belimumab; IS, immunosuppressants; PBO, placebo; PGA;
Physician Global Assessment; SD, standard deviation; SELENA-SLEDAI, Safety of
Estrogens in Lupus Erythematosus: National Assessment-Systemic Lupus
Erythematosus Disease Activity Index; SRI, SLE Responder Index
At Week 52, SRI improvement was
highest for belimumab 10 mg/kg (59%) compared with placebo in the steroids only
(49%) and steroids + AM (44%) groups. Overall, the incidence of AEs was
comparable between concomitant medication groups; the steroids + AM group had a
numerically higher percentage of serious AEs reported for belimumab (8%
placebo, 16% belimumab) compared with SoC.
Patients were not randomized by
concomitant medications group at baseline, therefore, subgroup size differed;
the study was not powered to examine differences in these concomitant
medication subgroups.
Conclusion: These data may be helpful in
understanding practice trends and the associated effects when belimumab is
added to a treatment regimen. Data interpretation is limited by the use of
baseline concomitant medication use, as over 52 weeks, treatment regimens may
have changed.
Disclosures: Study conducted and funded by GSK.
Katie White, PhD, Fishawack Indicia Ltd, UK, provided submission and editorial
assistance and was funded by GSK.
To cite this abstract in AMA style:
Schwarting A, Dooley MA, Roth D, Edwards L, Thompson A, Wilson B. Impact of Baseline Concomitant Medication Use on Belimumab Efficacy and Safety in Patients with Systemic Lupus Erythematosus (SLE) [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/impact-of-baseline-concomitant-medication-use-on-belimumab-efficacy-and-safety-in-patients-with-systemic-lupus-erythematosus-sle/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-baseline-concomitant-medication-use-on-belimumab-efficacy-and-safety-in-patients-with-systemic-lupus-erythematosus-sle/