Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Recent data suggest that patients with moderately active psoriatic arthritis (PsA) and a limited number of active joints have a high likelihood of achieving treatment goals with apremilast treatment (McInnes I, et al. Ann Rheum Dis. 2018;77(Suppl 2). Abstract 0927. Coates LC, et al. Arthritis Rheumatol. 2018;70(Suppl 10). Abstract 1607). Real-world evidence on the effect of apremilast on patients’ perception of the impact of their disease is limited. Here, we describe the effects of apremilast on the impact of disease in the real-world as measured by the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire in PsA patients with a swollen joint count (SJC) ≤4 at baseline.
Methods: The prospective, multicenter, observational REWARD study (NCT02875184; The Netherlands) is investigating apremilast treatment in real-world patients with PsA. Descriptive statistics were assessed for disease measures, including SJC (0-66) and tender joint count (TJC; 0-68), PsAID (0-10), and Patient’s Perception of Pain based on the visual analog scale (VAS; 0-100 mm) among patients with a limited number of swollen joints (SJC ≤4) at baseline. We report the effects of apremilast on these disease measures at treatment initiation and at the first 6 months of treatment with a data cutoff of December 2018.
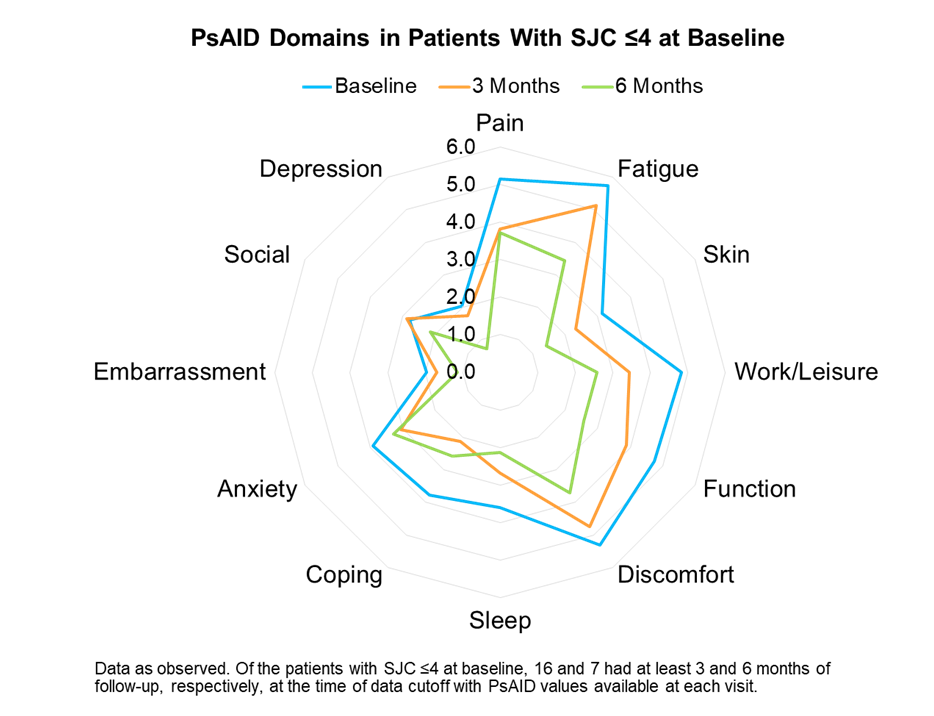
Results: A total of 48 patients from 9 clinics who were receiving apremilast were included in this interim analysis. Among these patients, 31 (65%) had SJC ≤4 and 17 (35%) had SJC >4 at baseline. Of the patients with SJC ≤4 at baseline, 18 and 8 had at least 3 and 6 months of follow-up, respectively, at the time of data cutoff. Results are reported for patients with available data at each time point. Baseline characteristics for patients with SJC ≤4 included a mean age of 54 years, mean body mass index of 29.1 kg/m2, and mean years since PsA diagnosis of 7.0 years; 58% of patients were female, 90% had prior cDMARD use, and 29% had prior biologic use. For patients with SJC ≤4, disease measures were reported as follows: The mean SJC at baseline, 3, and 6 months were 1.1, 0.6, and 0.1 and the mean TJC at baseline, 3, and 6 months were 4.5, 3.6, and 1.5, respectively. The mean PsAID scores at baseline, 3, and 6 months were 4.2, 3.4, and 2.6, respectively. Individual PsAID domain scores by time points are shown (Figure). Patients with a limited number of swollen joints showed gradual improvements in all domains of the PsAID. Mean Pain VAS scores at baseline, 2 weeks, 6 weeks, 3 months, and 6 months were 47, 48, 39, 29, and 26, respectively, in these patients.
Conclusion: Results from the real-world, prospective, multicenter, observational REWARD study suggest that PsA patients with a limited number of active swollen joints may benefit from apremilast treatment. Apremilast was associated with improvements in the perceived impact of disease, as observed by reductions in the PsAID and its core components.
To cite this abstract in AMA style:
Jansen T, Kroot E, van Vliet A, Pander J, Vis M. Impact of Apremilast on PsA Impact of Disease Core Components in Patients with a Limited Number of Active Joints: Results from a Real-World Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/impact-of-apremilast-on-psa-impact-of-disease-core-components-in-patients-with-a-limited-number-of-active-joints-results-from-a-real-world-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-apremilast-on-psa-impact-of-disease-core-components-in-patients-with-a-limited-number-of-active-joints-results-from-a-real-world-study/