Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: The morbidity associated with the use of immunosuppressants is well described in patients (pts) with autoimmune ophthalmic disease and presents a challenge to treating physicians. The purpose of this analysis was to determine the impact of adalimumab (ADA) on immunomodular (IMM) use in pts with active and inactive non-infectious uveitis in the ongoing open label study: VISUAL-III.
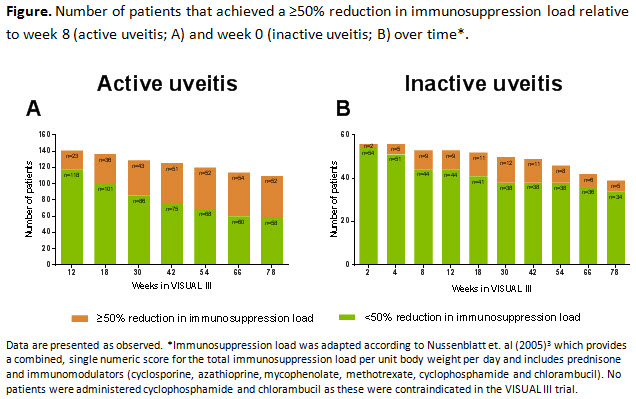
Methods: Adults who met treatment failure criteria1,2 in VISUAL-I/VISUAL-II studies (defined as pts with active uveitis) or who successfully completed the parent studies without treatment failure (defined as pts with inactive uveitis) were eligible to enroll in VISUAL-III and received ADA 40-mg every other week for the study duration. Corticosteroids (CS) and/or IMM therapy were permitted as needed. For this analysis, proportion of pts on IMM and percent change in systemic IMM dose were analyzed in pts using medications for immunosuppression. Interim follow-up data from VISUAL-III baseline (week 0) through week-78 are described. In addition, proportion of pts who achieved a ≥50% reduction in immunosuppression load (CS + IMM) relative to week 8 (for active uveitis) and relative to week 0 (for inactive uveitis) is reported. Data are presented as-observed. Adverse events are reported from first ADA dose up to the data cut-off date of 31-October-2016.
Results: Of 424 pts enrolled, 371 (active uveitis; n=242 and inactive uveitis; n=129) were included in the intent-to-treat analysis. Of these 371 pts, 117 (32%) (active uveitis; n=65 (56%) and inactive uveitis; n=52 (44%)) were using IMM at VISUAL-III entry. By week 78, the number of pts using IMMs decreased (active uveitis, n=36 and inactive uveitis, n=32). The systemic IMM dose was substantially reduced by week 78 compared to baseline in pts with active and inactive uveitis. In pts with active uveitis using CS + IMM, 47% (52/110) achieved a ≥50% reduction in immunosuppression load relative to week 8, while in pts with inactive uveitis 13% (5/39) achieved a ≥50% reduction in immunosuppression load relative to week 0 (Figure A-B). Adverse Event rates were consistent with previous VISUAL trials.
Conclusion: Long-term treatment with ADA suggests decreased IMM dependence along with substantial reduction in IMM dose in pts with active and inactive uveitis.
References:
1. Jaffe GJ et. al (2016). NEJM; 375:932-943.
2. Nguyen QD et. al (2016). The Lancet; 388(10050): 1183-1192.
3. Nussenblatt et. al (2005). Ophthalmology; 112 (5):764-770.
To cite this abstract in AMA style:
Schwartzman S, Van der Horst-Bruinsma I, Adan A, Goto H, Kamoi K, Kron M, Song AP, Douglas K, Pathai S, Foster CS. Impact of Adalimumab on Immunosuppressant Use in Patients with Active and Inactive Non-Infectious Intermediate, Posterior, or Pan-Uveitis in the Ongoing Open Label Study: Visual-III [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/impact-of-adalimumab-on-immunosuppressant-use-in-patients-with-active-and-inactive-non-infectious-intermediate-posterior-or-pan-uveitis-in-the-ongoing-open-label-study-visual-iii/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-adalimumab-on-immunosuppressant-use-in-patients-with-active-and-inactive-non-infectious-intermediate-posterior-or-pan-uveitis-in-the-ongoing-open-label-study-visual-iii/