Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: The APS ACTION Registry was created to study the natural course of antiphospholipid syndrome (APS) over 10 years in persistently antiphospholipid antibody (aPL) positive patients with or without systemic autoimmune diseases (SAIDx). Given data to support the role of immunosuppression (IS) in the management of APS patients with certain clinical phenotypes, e.g, diffuse alveolar hemorrhage (DAH), our primary objective was to characterize IS use in aPL-positive patients without other SAIDx.
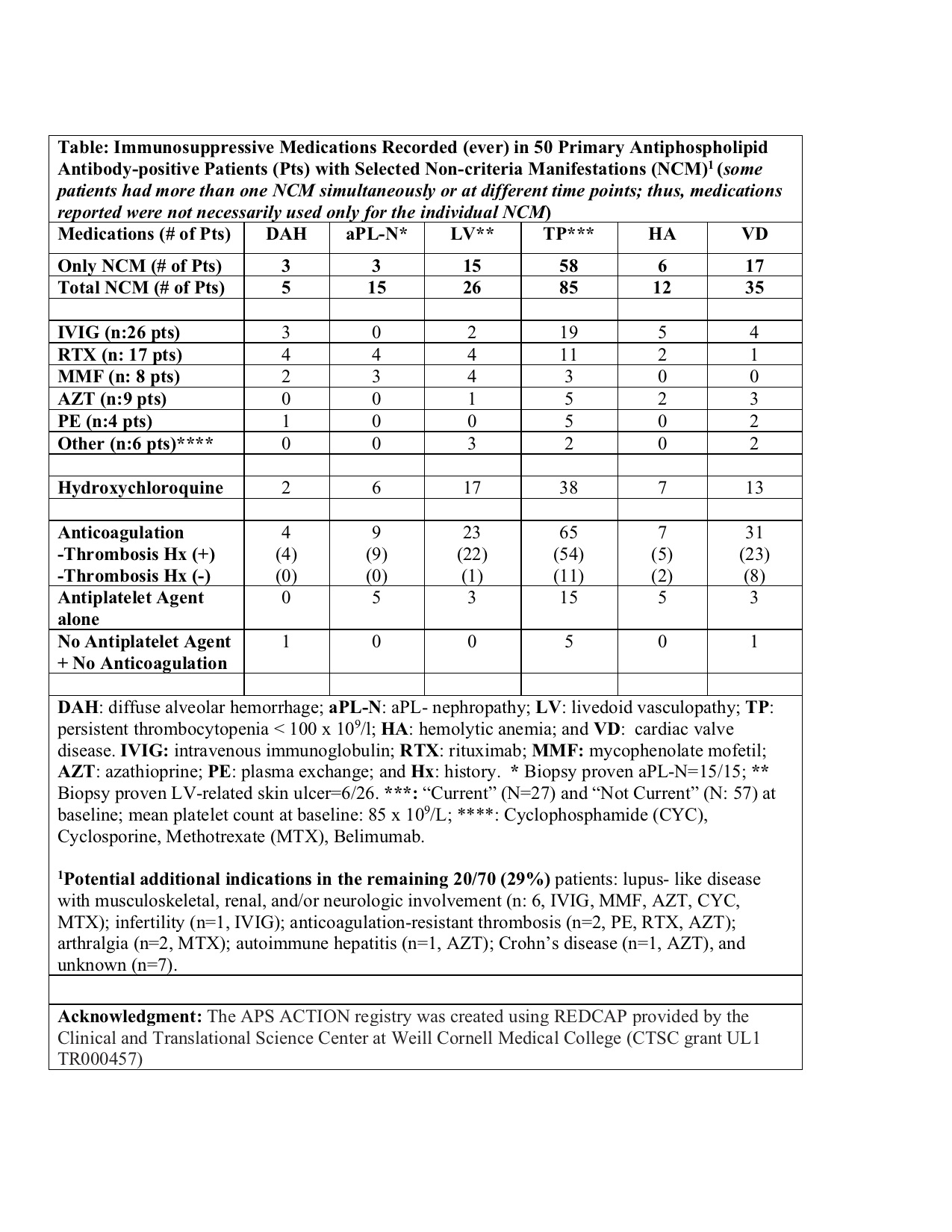
Methods: A central online database was used to collect detailed clinical data. The inclusion criteria were positive aPL based on the laboratory section of the current APS Classification Criteria, tested at least twice within one year prior to enrollment. For this descriptive analysis, we only included aPL-positive patients without other SAIDx and excluded those with catastrophic APS (CAPS). We retrieved data on demographics, aPL/APS-related history including selected non-criteria manifestations (DAH, antiphospholipid-nephropathy [aPL-N], livedoid vasculopathy-related skin ulcers [LV], thrombocytopenia [TP], hemolytic anemia [HA], and cardiac valve disease [VD]); and IS use (ever) (Table).
Results: As of 1/2021, 866 patients were included in the registry; 325 (38%) were excluded due to another SAIDx and an additional five due to CAPS. Of the remaining 536 patients (mean age at entry: 45±13y; 70% female; 70% white; 432 [81%] meeting the APS Classification; and 143 [27%] with at least one selected non-criteria manifestations), 70 (13%) used IS (ever) excluding corticosteroids (CS) and hydroxychloroquine (HCQ). Of 70 IS users (non-CS/HCQ), 50 (71%) had at least one of the selected non-criteria manifestations. Of 143 patients with at least one of the selected non-criteria manifestations, 38 (27%) had no history of thrombosis; 19/38 (50%) received anticoagulation with/without antiplatelets, 15/38 (39%) antiplatelets alone, and 4/38 (11%) no antithrombotic agents. Four of 5 (80%) DAH patients, 6/15 (40%) aPL-N, 10/26 (39%) LV, 32/85 (38%) TP, 7/12 (58%) HA, and 9/35 (26%) VD patients were reported to receive non-CS/HCQ immunosuppression (Table).
Conclusion: In our multi-center international cohort, 13% of aPL-positive patients without other systemic autoimmune diseases, mostly those with selected non-criteria manifestations, were reported to use immunosuppressives other than corticosteroids and hydroxychloroquine. Given the inconsistent reporting of immunosuppression use in aPL-positive patients with non-criteria manifestations, systematic studies are urgently needed to better define the role of immunosuppression for different aPL-related non-criteria manifestations.
To cite this abstract in AMA style:
Erton Z, Karp- Leaf R, Andrade D, Tektonidou M, Pengo V, Sciascia S, Ugarte A, Belmont H, Gerosa M, Fortin P, lopez-pedrera C, Ji L, Atsumi T, Cohen H, de Jesus G, Branch D, Nalli C, Kello N, Petri M, Rodriguez-Almaraz E, Cervera R, Knight J, Artim-Esen B, Willis R, Bertolaccini M, Roubey R, Erkan D, APS ACTION o. Immunosuppression Use in Primary Antiphospholipid Antibody Positive Patients: Descriptive Analysis of the AntiPhospholipid Syndrome Alliance for Clinical Trials and InternatiOnal Networking (APS ACTION) Clinical Database and Repository (“Registry”) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/immunosuppression-use-in-primary-antiphospholipid-antibody-positive-patients-descriptive-analysis-of-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-actio/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunosuppression-use-in-primary-antiphospholipid-antibody-positive-patients-descriptive-analysis-of-the-antiphospholipid-syndrome-alliance-for-clinical-trials-and-international-networking-aps-actio/