Session Information
Date: Saturday, November 6, 2021
Title: Plenary I (0453–0457)
Session Type: Plenary Session
Session Time: 11:45AM-12:00PM
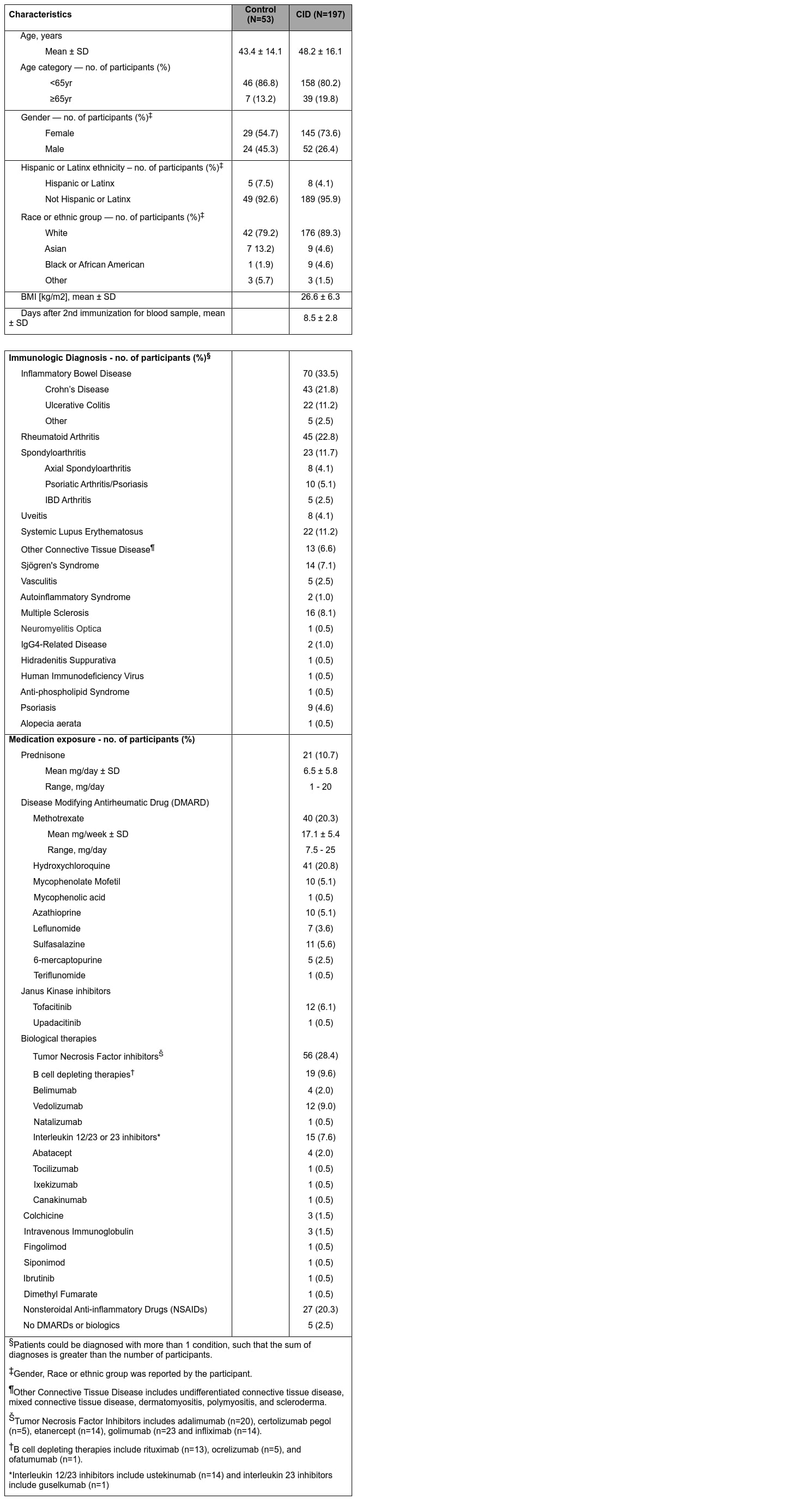
Background/Purpose: Individuals with chronic inflammatory diseases (CID) are frequently treated with immunosuppressive medications that can increase their risk of severe COVID-19. While novel mRNA-based SARS-CoV-2 vaccination platforms provide robust protection in immunocompetent individuals, the immunogenicity in CID patients. Therefore, determining the effectiveness of SARS-CoV-2 vaccines in these patients is essential to risk-stratify them with impaired protection and provide clinical guidance regarding medication management.
Methods: We initiated the COVID-19 Vaccine Responses in Patients with Autoimmune Disease (COVaRiPAD) study, a prospective assessment of mRNA-based vaccine immunogenicity and reactogenicity in patients with CID. We collected blood from 197 adults with CIDs and 53 immunocompetent controls before initial immunization and 1-2 weeks after the second immunization. Serum anti-SARS-CoV-2 spike (S) IgG+ binding and neutralizing antibody titers were quantified to assess the magnitude and quality of the humoral response following vaccination.
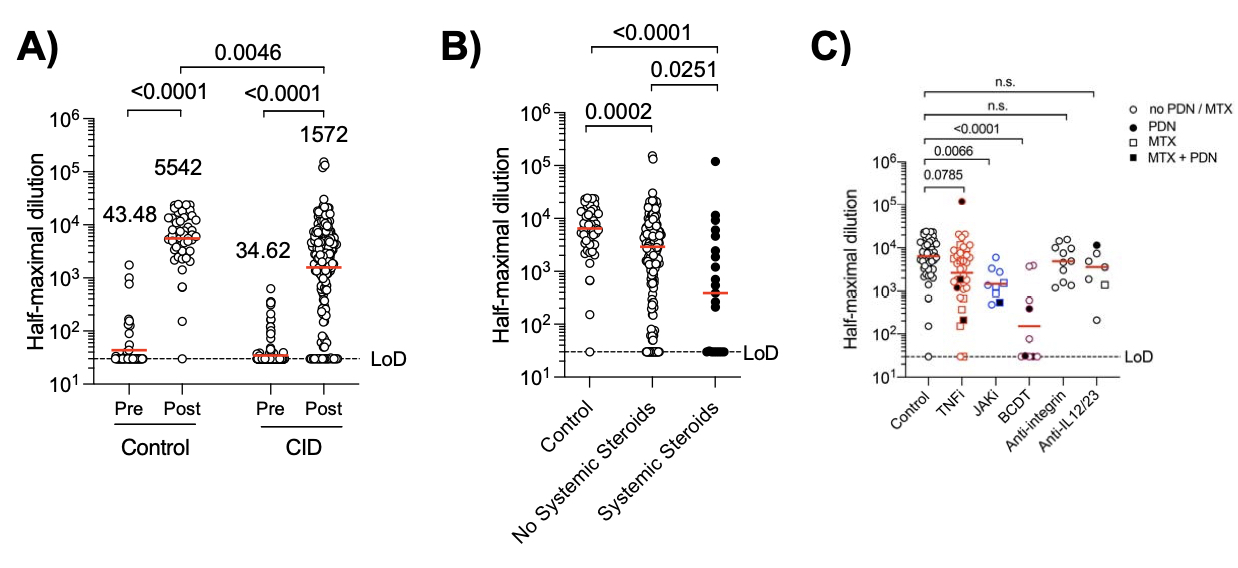
Results: Compared to immunocompetent controls, a three-fold reduction in anti-S IgG titers (P=0.0046) and SARS-CoV-2 neutralization (P< 0.0001) to the D614G common variant were observed in CID patients. B cell depletion and glucocorticoids exerted the strongest effect with a 36- and 13-fold reduction in humoral responses, respectively (p< 0.0001). Janus kinase inhibitors and antimetabolites, including methotrexate, also blunted antibody titers in multivariate regression analyses (P< 0.0001, P=0.0023, respectively). Other targeted therapies, such as TNF inhibitors, IL-12/23 inhibitors, and integrin inhibitors, had only modest impacts on antibody formation and neutralization.
Conclusion: CID patients treated with immunosuppressive therapies exhibit impaired SARS-CoV-2 vaccine-induced immunity, with glucocorticoids and B cell depletion therapy more severely impeding optimal responses. We are currently determining cross-variant neutralization, long-term antibody and neutralization titers, and T cell responses in this cohort.
To cite this abstract in AMA style:
Paley M, Deepak P, Kim W, Yang M, Carividi A, Demissie E, El-Qunni, A, Haile A, Huang K, Kinnett B, Liebeskind M, Liu Z, McMorrow L, Paez D, Pawar N, Perantie D, Schriefer R, Sides S, Thapa M, Akuse S, Burdess S, Rose A, Mitchell L, Chahin S, Ciorba M, Graf J, Katz P, Matloubian M, O'Halloran J, Presti R, Wu G, Whelan S, Buchser W, Gensler L, Nakamura M, Ellebedy A, Kim A. Immunosuppression Attenuates Antibody and Neutralization Titers in Patients with Chronic Inflammatory Disease Following SARS-CoV-2 Vaccination [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/immunosuppression-attenuates-antibody-and-neutralization-titers-in-patients-with-chronic-inflammatory-disease-following-sars-cov-2-vaccination/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunosuppression-attenuates-antibody-and-neutralization-titers-in-patients-with-chronic-inflammatory-disease-following-sars-cov-2-vaccination/