Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: A better understanding of the immunological differences between psoriatic arthritis (PsA) patients (pts) who are tumor necrosis factor inhibitor (TNFi)-naïve and who have inadequate response to TNFi (TNFi-IR) may guide treatment choices. In DISCOVER-1, benefit of the IL-23p19 subunit inhibitor guselkumab (GUS) every-four-weeks (Q4W) and Q8W over placebo (PBO) in improving PsA signs and symptoms was seen in adults with active PsA.1 The Phase 3b COSMOS study of GUS Q8W vs PBO in TNFi-IR PsA pts corroborated these findings.2 We assessed baseline (BL) molecular differences between TNFi-naïve and TNFi-IR PsA pts and investigated GUS pharmacodynamic effect on cytokine expression over time in these cohorts.
Methods: Serum samples collected from consenting biomarker substudy pts in DISCOVER-11 (TNFi-naïve [n=101] and TNFi-IR [n=17]), DISCOVER-23 (TNFi-naïve [n=150]), and COSMOS2 (TNFi-IR [n=76]) were analyzed for selected serum cytokine levels. TNFi-IR pts in this post-hoc analysis had active PsA and discontinued 1-2 TNFi due to inadequate efficacy; these pts required a TNFi-specific washout period prior to starting GUS. Pharmacodynamic effect of GUS Q8W on cytokine levels was assessed. Differential BL cytokine expression, associations between BL cytokine levels and clinical response (Psoriasis [PsO] Area and Severity Index 75% improvement from BL [PASI75] and American College of Rheumatology 20% improvement [ACR20]), and GUS effect on cytokine levels were analyzed with a General linear model and Spearman linear regression.
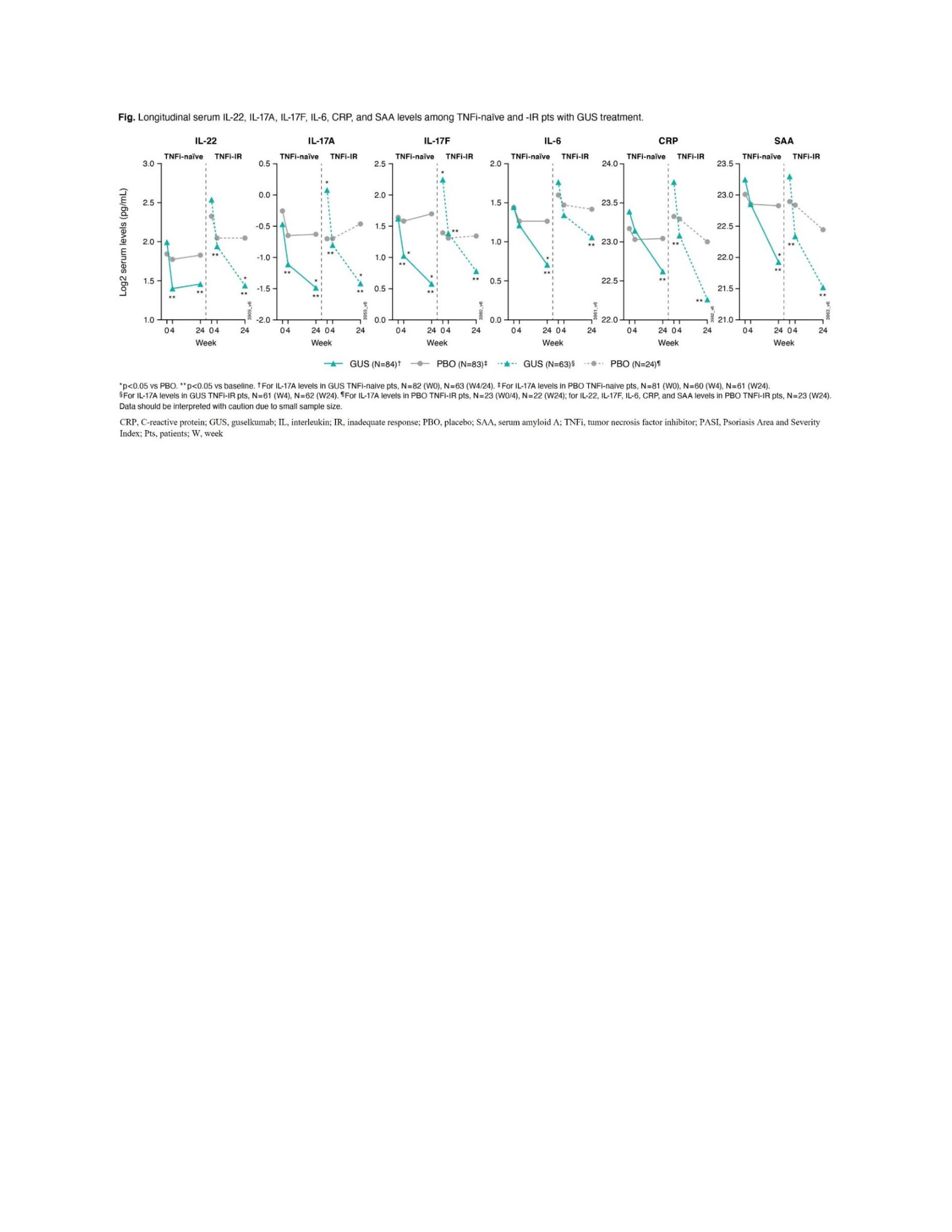
Results: BL pt demographics, disease characteristics, and conventional synthetic disease-modifying antirheumatic drug (csDMARD) use were comparable between TNFi-naïve (DISCOVER-1 and DISCOVER-2, N=251) and TNFi-IR (DISCOVER-1 and COSMOS, N=93) pts, with differences in mean PASI score (8.9 v 12.5), swollen joint count (SJC) (11.7 v 10.3), PsA duration (5.8 v 9.8 years), and PsO duration (16.7 v 20.4 years; Table). BL serum IL-22 and TNFα levels for pooled treatment groups were higher in TNFi-IR than TNFi‑naïve pts (p< 0.05). At W24, GUS reduced IL-22, IL-17A, IL-17F, IL-6, C-reactive protein (CRP), and serum amyloid A protein to similar levels in both cohorts (p< 0.05; Figure). W24 PASI75 responders in GUS arm had higher BL IL-17F levels in both cohorts (p< 0.05) and higher IL-22 levels in TNFi-IR pts only (p< 0.05). A trend of upregulated BL IL-22 expression in W24 ACR20 responders was seen for TNFi-IR pts treated with GUS (p=0.07).
Conclusion: Elevated BL IL-22 expression and association between BL IL-22 levels and W24 PASI75 response, and a W24 trend for an association between upregulated BL IL-22 and ACR20 response, in TNFi-IR pts seen in this exploratory analysis may suggest increased involvement of the IL-23 pathway in TNFi-IR pts. GUS showed comparable and significant pharmacodynamic effects for TNFi-naïve and TNFi-IR pts, consistent with observed clinical responses.
References:
1Deodhar A, et al. Lancet. 2020;395:1115-25
2Coates LC, et al. Ann Rheum Dis. 2021;80:140-1
3Mease P, et al. Lancet. 2020;395:1126-36
To cite this abstract in AMA style:
Siebert S, Coates L, Schett G, P. Raychaudhuri S, Chen W, Gao S, Chakravarty S, Shawi M, Lavie F, Theander E, Neuhold M, Kollmeier A, Xu X, Rahman P, Mease P, Deodhar A. Immunological Differences Between PsA Patients Who Are Tumor Necrosis Factor Inhibitor-Naïve and Who Have Inadequate Response to Tumor Necrosis Factor Inhibitors [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/immunological-differences-between-psa-patients-who-are-tumor-necrosis-factor-inhibitor-naive-and-who-have-inadequate-response-to-tumor-necrosis-factor-inhibitors/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immunological-differences-between-psa-patients-who-are-tumor-necrosis-factor-inhibitor-naive-and-who-have-inadequate-response-to-tumor-necrosis-factor-inhibitors/