Session Information
Date: Monday, November 13, 2023
Title: (1052–1081) Immunological Complications of Medical Therapy Poster
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Immune checkpoint inhibitors (ICI) improve overall survival in many cancer patients by activating their immune system. However, they can cause off-target immune-related adverse events (irAEs) including inflammatory arthritis (ICI-IA), sometimes prompting ICI discontinuation. Another course of ICI is now often considered in patients with cancer progression and previous irAEs, but there are limited data regarding the safety of ICI rechallenge in this context1. We aimed to assess the rate and clinical features associated with ICI-IA flare/recurrence upon ICI rechallenge.
Methods: We conducted a multicenter observational study including cancer patients with ICI-IA (defined by either the presence of at least one synovitis on physical exam and/or on imaging or polymyalgia rheumatica (PMR)-like symptoms) who started a second course of ICI more than 3 months after ICI discontinuation in 4 french university hospitals. Patients with a follow-up of < 3 months after ICI rechallenge were excluded. Charts were manually reviewed to extract data on baseline characteristics, investigations, management and both ICI-IA and tumor outcomes. Patients who experienced a flare or recurrence of ICI-IA upon rechallenge were compared to those who did not.
Results: 23 patients were included with a median follow-up duration of 7 months from ICI rechallenge. The most frequent ICI-IA presentations were PMR-like (n=11, 48%) and symmetric polyarthritis (n=7, 30.4%), which occurred after a mean time of 6.6 months during the first course of ICI. Almost all patients were treated with prednisone (n=20, 87%), 2 with csDMARDs (8.7%) and 1 with biologics (4.3%). Reason for ICI discontinuation was ICI-IA (n=8; 35%), cancer progression (n=7; 30%), other irAE (n=4; 17%) and cancer stable or in remission (n=3; 13%).
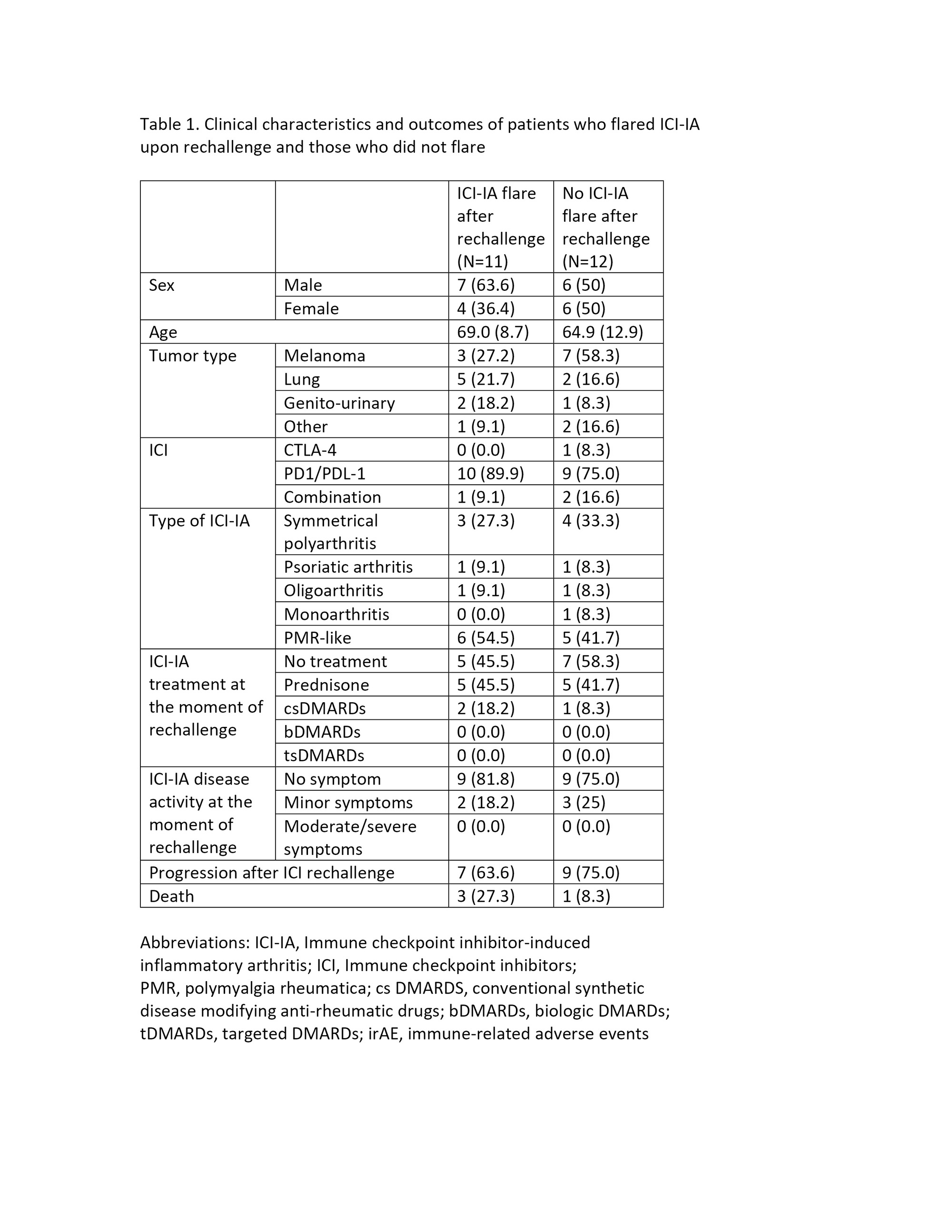
Reason for ICI rechallenge was cancer progression in all patients. At the time of ICI rechallenge, 18 patients reported no symptoms of ICI-IA (78%), 5 had minor symptoms (22%) and none had moderate or severe symptoms. Regarding baseline ICI-IA treatment, 12 patients (52%) were not receiving any ICI-IA treatment at the time of ICI rechallenge, 10 (43%) were still on prednisone and 3 (13%) were on csDMARDs. ICI-IA flare/recurrence occurred in 12 patients (52%) with a mean time of 2.2 months after ICI rechallenge. Only one patient discontinued ICI due to ICI-IA recurrence. When comparing patients who experienced ICI-IA flare/recurrence with those who did not, there was no difference in terms of baseline ICI-IA treatment or disease activity (Table 1). Laboratory and imaging data are currently being analyzed.
Conclusion: In this first observational study of ICI-IA patients rechallenged with ICI, about half of the patients experienced ICI-IA flare/recurrence which occurred earlier than the first episode of ICI-IA but allowing ICI continuation in all but one patient. Patients still on prednisone or csDMARDs to control their ICI-IA at the time of rechallenge did not seem at increased risk of flaring. Larger studies are needed to clarify risk factors of ICI-IA flare with ICI rechallenge.
References:
1. Dolladille C. et al., JAMA Oncology 2020
To cite this abstract in AMA style:
Ladouceur A, Mouterde G, TISON A, Bitoun S, Mary-Prey S, Dutriaux C, Gerard E, Pham-Ledard A, Beylot-Barry M, Zysman M, Veillon R, Domblides C, Daste A, Gross-Goupil M, Sionneau B, Lefort F, Larroquette M, Barnetche T, Richez C, Truchetet M, Schaeverbebke T, Kostine M. Immune Checkpoint Inhibitor Rechallenge in Patients Who Previously Experienced Immune-Related Inflammatory Arthritis: A Multicenter Observational Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/immune-checkpoint-inhibitor-rechallenge-in-patients-who-previously-experienced-immune-related-inflammatory-arthritis-a-multicenter-observational-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immune-checkpoint-inhibitor-rechallenge-in-patients-who-previously-experienced-immune-related-inflammatory-arthritis-a-multicenter-observational-study/