Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: The advent of immune checkpoint inhibitor (ICI) therapy has revolutionized the treatment of cancer. Patients with pre-existing autoimmune diseases (AID) were largely excluded from ICI clinical trials, due to concerns related to enhanced immune-related toxicity. Real world evidence is required to understand the effectiveness and safety of ICI therapy in individuals with pre-existing AID.
Methods: We performed a single institution retrospective study of patients exposed to ICI therapy between 2012 and 2019 at CancerCare Manitoba (Winnipeg, Canada). Pharmacy records were used to determine ICI exposure. A chart review of the electronic health record was conducted to identify patients with pre-existing AID. Baseline clinical and oncologic characteristics were collected. The primary outcome of interest was immunotoxicity, defined as either a flare of the pre-existing AID or a new immune related adverse event (irAE). The AID group was matched (1:2) to an ICI-exposed group without AID based on age, sex, and cancer type. We used logistic regression modeling to examine the association between AID status and immunotoxicity.
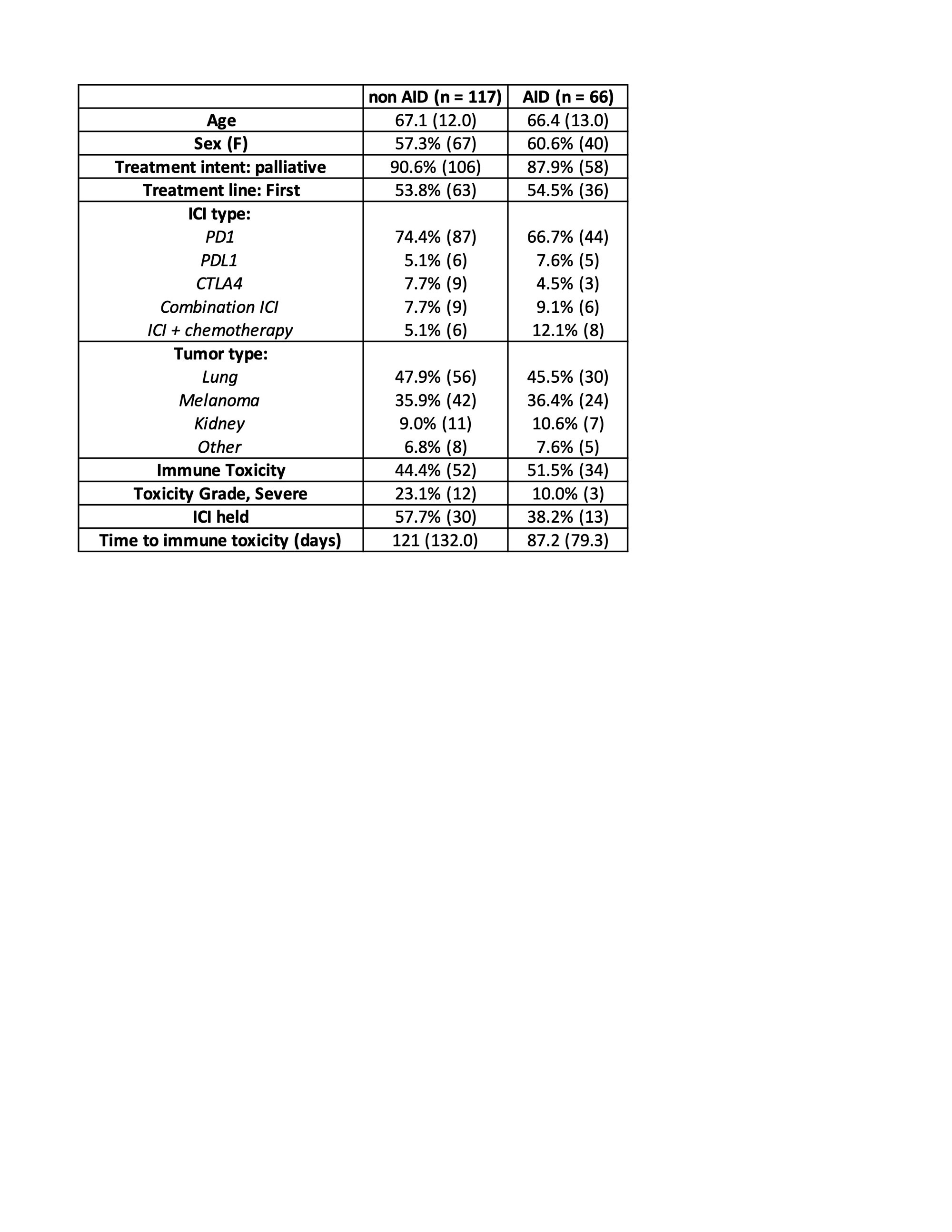
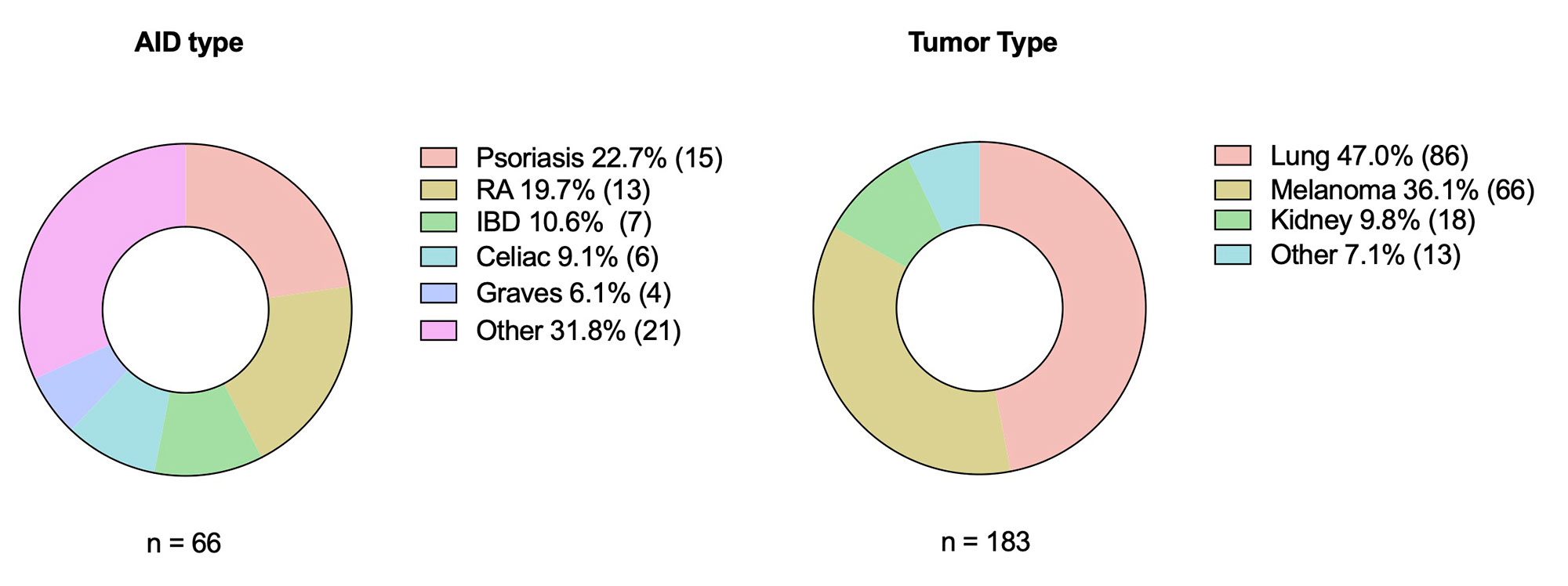
Results: We identified a total of 813 patients exposed to ICI therapy during the study period. Within this cohort, 8.1% (N=66) had a pre-existing AID. The most common type of AID was psoriasis (22.7%, N=15), followed by rheumatoid arthritis (19.7%, N=13). 9 patients (13.6%) were on baseline prednisone or DMARD therapy at time of ICI start. Most received monotherapy with a PD-1 inhibitor (N=44, 66.7%). Rate of immunotoxicity was 51.5%, with 15.1% (N=10) experiencing a flare of the AID, 46.9% (N=31) experiencing a new irAE, and 10.6% (N=7) experiencing both a flare and a new irAE. Of those with an irAE, 90% were low grade (grade 1 or 2). When matched to a cohort without pre-existing AID (N=117), rates of irAE’s were similar (44.4% in the non-AID group vs. 46.9 % in the AID group, p=0.742), with no apparent difference in clinical severity. After controlling for type of ICI and cancer type, there was no significant association between AID status and irAE (odds ratio=1.07, 95% CI 0.582-1.98, p=0.822).
Conclusion: In this retrospective cohort study, pre-existing AID was not associated with increased rates of irAE’s as compared to a matched control group without AID, even after adjusting for type of ICI therapy received. Within the AID group, there was a relatively low rate of disease flare. These results should be confirmed in prospective studies. Future analyses of this cohort will examine oncologic outcomes between the AID and non-AID groups.
To cite this abstract in AMA style:
Raghavan A, O'Neil L, Ye C, Graham J. Immune Checkpoint Inhibition and Preexisting Autoimmune Disease: A Retrospective Cohort Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/immune-checkpoint-inhibition-and-preexisting-autoimmune-disease-a-retrospective-cohort-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/immune-checkpoint-inhibition-and-preexisting-autoimmune-disease-a-retrospective-cohort-study/