Session Information
Session Type: Abstract Submissions (ACR)
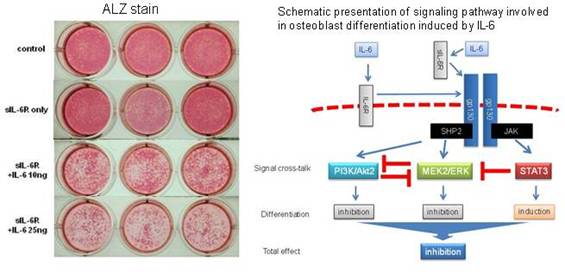
Background/Purpose: Interleukin-6 (IL-6), a potent inflammatory cytokine, plays a key role in the pathogenesis of rheumatoid arthritis (RA), including osteoporosis not only in inflamed joints but possibly also in whole body. Since increase of bone formation markers is recognized in RA patients treated with tocilizumab, a humanized anti- IL-6 receptor antibody, IL-6 could be thought to have negative effect on osteoblast differentiation. However, previous reports regarding the effects of IL-6 on osteoblast differentiation in vitro are not consistent. IL-6 activates two major intracellular signaling pathways, SHP2/MEK/ERK and JAK/STAT3, and can also lead to the activation of additional signaling cascade involving PI3K/Akt. The purpose of this study was to clarify the effect of IL-6 on osteoblast differentiation in vitro, with consideration of intracellular signaling pathways. Cross-talk between these pathways was also investigated.
Methods: Osteoblast differentiation was induced in murine MC3T3-E1 osteoblastic cells and primary calvarial osteoblasts with or without addition of IL-6 (soluble IL-6 receptor was used to enhance the effect of IL-6). Osteoblast differentiation was assessed by alkaline phosphatase (ALP) activity, mineralization and expression of osteoblastic gene. We examined which signaling pathways were activated by IL-6 and their effects on the differentiation were assessed by using each specific inhibitor and each knockdown.
Results: IL-6 significantly reduced ALP activity, mineralization and expression of osteoblastic gene, in a dose-dependent manner, which indicates a negative effect of IL-6 on osteoblast differentiation. Signal transduction study demonstrated that IL-6 activated not only two major signaling pathways, SHP2/MEK/ERK and JAK/STAT3, but also SHP2/PI3K/Akt2 signaling pathway. The negative effect of IL-6 on osteoblast differentiation was restored by inhibition of MEK as well as PI3K, while it was enhanced by inhibition of STAT3. Knockdown of MEK2 and Akt2 transfected with siRNA enhanced ALP activity and gene expression of Runx2. These results indicate that IL-6 negatively regulates osteoblast differentiation through SHP2/MEK2/ERK and SHP2/PI3K/Akt2 pathways, while it effects positively through JAK/STAT3. Moreover, STAT3 inhibitor enhanced IL-6-induced phosphorylation of ERK. MEK inhibitor enhanced IL-6-induced phosphorylation of Akt. PI3K/Akt inhibitor enhanced IL-6-induced phosphorylation of ERK. IL-6-induced ERK and Akt signaling pathways, both of which are downstream of SHP2, could negatively regulate each other reciprocally.
Conclusion: IL-6 could negatively regulate osteoblast differentiation through SHP2/MEK2/ERK and SHP2/PI3K/Akt2 pathways, while it effects positively through JAK/STAT3. Inhibition of MEK2 and Akt2 signaling in osteoblasts might be of potential use in the treatment of osteoporosis in RA.
Disclosure:
S. Kaneshiro,
None;
K. Ebina,
None;
K. Shi,
None;
C. Higuchi,
None;
H. Yoshikawa,
None;
J. Hashimoto,
None;
M. Hirao,
None.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/il-6-suppresses-osteoblast-differentiation-through-the-shp2mek2-and-shp2akt2-pathways-in-vitro/