Session Information
Date: Monday, November 11, 2019
Title: 4M119: Sjögrenʼs Syndrome – Basic & Clinical Science (1902–1907)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: IL-6 is suspected to play an important pathogenic role in primary Sjögren’s syndrome (pSS) through its crucial roles in B-cell activation, and T-cell polarization, as shown in mice models and ex vivo studies. To investigate the relevance of IL-6 as a therapeutic target in patients with pSS, we performed a double- blind randomized placebo-controlled trial evaluating tocilizumab.
Methods: Inclusion criteria were pSS according to AECG criteria and an ESSDAI (score of systemic complications) ≥ 5. Patients received 6 monthly infusions of tocilizumab or placebo. Primary endpoint criteria was response to treatment evaluated at week 24. Response to treatment was defined by the combination of i) a decrease of at least 3 points in ESSDAI ; ii) no occurrence of moderate or severe activity in any new domain of the ESSDAI compared to enrollment ; iii) absence of worsening in physician’s global assessment on visual numeric scale ≥ 1/10. Secondary endpoints included change in ESSPRI (mean of patient’s fatigue, pain and dryness visual analogic scales), and in Schirmer’s test. The data were analysed using Bayesian methods on an intent-to-treat basis.
Results: 55 patients (women : 98.2%, mean age : 50.9 [26 ; 76] years, anti-SSA antibody-positive : 84.7%) were randomized to tocilizumab and 55 patients to placebo (women : 90.3%, mean age : 54.9 [30 ; 80] years, anti-SSA positive : 87.6%). Mean ESSDAI was 11.5 [5 ; 25] and 12.4 [5 ; 39] and mean ESSPRI was 6.4 [2 ; 9] and 6.4 [1 ; 9] in the tocilizumab and in the placebo group, respectively.
The results on the primary outcome criteria were similar in both groups : 54.2% [41.3 ; 66.7%] of responders in the tocilizumab group, 62.1 %[49.0 ; 74.1] in the placebo group, OR = 1.6 [0.3 ; 3.3]
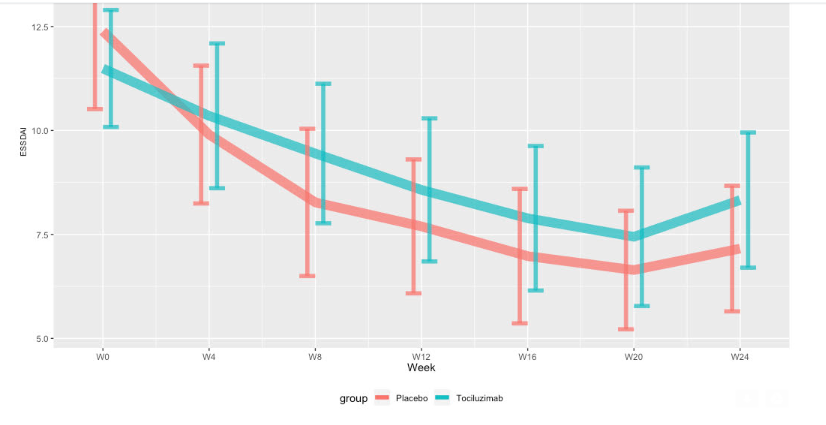
Mean ESSDAI at week 24 were 6.6 [4.7 ; 9.0] and 5.4 [3.7 ; 7.6] in the tocilizumab and placebo group, respectively, with a similar difference of the changes from baseline between groups (interaction, 0.9 [-1.3 ; 3.2]) (Figure 1).
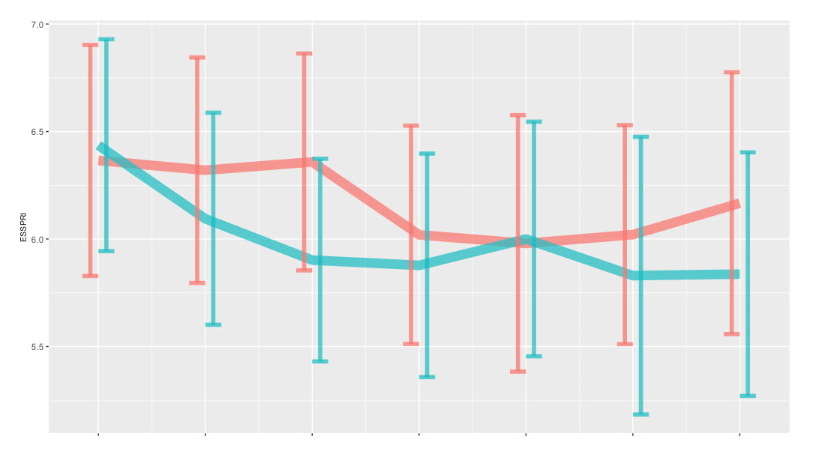
Mean ESSPRI at week 24 was 6.0 [5.0 ; 7.0] and 6.2 [5.2 ; 7.1] in the tocilizumab and placebo group respectivey, with a similar difference of the changes from baseline between groups (0.1 [-0.4 ; 0.7]) (Figure 2).
Change in Schirmer’s test was similar in both groups.
Change from baseline in number of tender and swolllen joints was higher in the tocilizumab (from 7.4 [6.7 ; 8.1] to 3.6 [3.3 ; 3.9] and from 2.2 [1.8 ; 2.6] to 0.6 [0.5 ; 0.7], respectively) than in the placebo group (from 7.8 [7.1 ; 8.6] to 4.9 [4.6 ; 5.3] and from 2.4 [2.0 ; 2.8] to 1.3 [1.1 ; 1.5]) (interaction 1.2 [0.2 ; 2.7] and 0.8 [0.3 ; 1.4], respectively). Number of severe adverse events was similar in the 2 groups.
Conclusion: In this randomized placebo-controlled study, tocilizumab did not reach its primary outcome criteria and had no impact on main symptoms in pSS. Some improvement was observed in patients with articular involvement. Effect of tocilizumab on other subsets of patients and on immunological parameters is currently investigated and will be reported at ACR.
To cite this abstract in AMA style:
Felten R, Meyer N, Duffaut P, Saadoun D, Hachulla E, Hatron P, Salliot C, Perdriger A, Morel J, Mekinian A, Vittecoq O, Berthelot J, Dernis E, Le Guern V, Dieudé P, Larroche C, Richez C, Martin T, Zarnitsky C, Blaison G, Kieffer P, Maurier F, Rist S, Cacoub P, Andres E, Chatelus E, Sordet C, Sibilia J, Arnold C, Tawk M, Aberkane O, Seror R, Holterbach L, Mariette X, Gottenberg J. IL-6 Receptor Inhibition in Primary Sjögren Syndrome : Results from a Randomized Multicenter Academic Double Blind Placebo-controlled Trial of Tocilizumab in 110 Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/il-6-receptor-inhibition-in-primary-sjogren-syndrome-results-from-a-randomized-multicenter-academic-double-blind-placebo-controlled-trial-of-tocilizumab-in-110-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/il-6-receptor-inhibition-in-primary-sjogren-syndrome-results-from-a-randomized-multicenter-academic-double-blind-placebo-controlled-trial-of-tocilizumab-in-110-patients/