Session Information
Date: Tuesday, November 14, 2023
Title: (2039–2060) Pediatric Rheumatology – Clinical Poster III: Potpourri
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
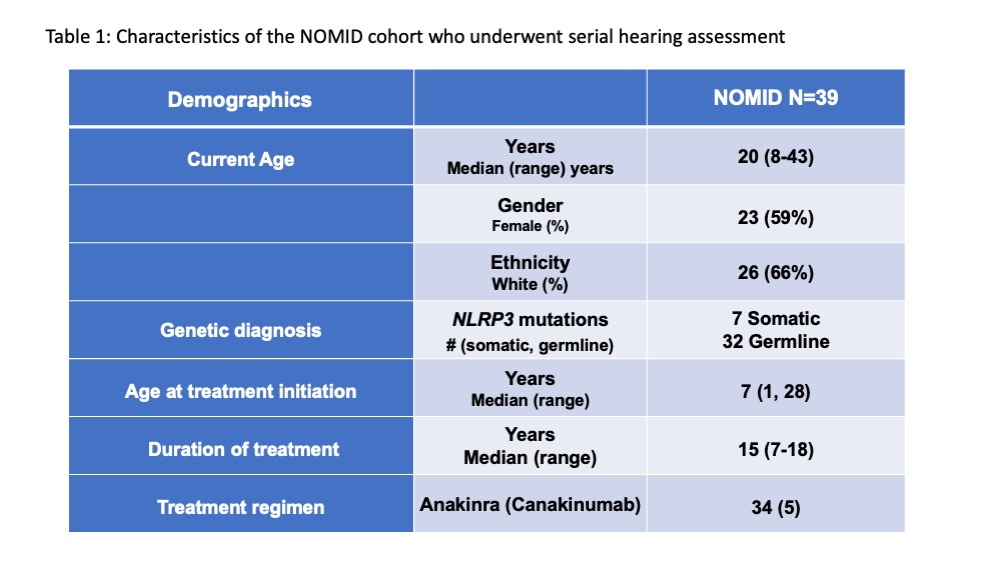
Background/Purpose: NOMID is a severe form of cryopyrin-associated periodic syndrome characterized by systemic inflammation and CNS manifestations, including sensorineural hearing loss. IL-1 blocking agents, Anakinra and canakinumab, are standard treatment, we assessed the long-term impact of IL-1 blocking treatment on progression of hearing loss.
Methods: Pts (n=39) enrolled in NCT02974595 protocol underwent hearing tests at baseline and on treatment (n=447) Table 1. Bone conduction at four frequency (4F-PTA) (average hearing at 500, 1000, 2000, 4000 Hz) and bone conduction at 4000 Hz only (surrogate for high frequency (HF) hearing) were longitudinally evaluated. 4F-PTA >25 dB, or HF >25 dB at baseline were defined as baseline 4F-PTA or HF hearing loss respectively.
Progressive 4F-PTA hearing loss was defined as 20dB increase in one frequency or 10dB increase in two consecutive frequencies and progressive HF hearing loss as change from baseline by 10dB. Pts’ ears were analyzed separately as ‘good’ and ‘bad,’ where hearing levels differed. Pts with a hearing level of >80 dB were excluded from trajectory analysis. Incidences of upper respiratory infections were extracted from records and brain MRI scored for cochlear enhancement. 36 or 39 pts. had a lumbar puncture at their last visit; 65 biomarkers were measured in CSF and compared with three healthy controls.
Linear mixed models were applied to trajectories of hearing loss before and after treatment. Multivariate and univariate logistic regression assessed the impact of baseline hearing loss and infections on progression and of CSF inflammatory cytokines on MRI cochlear enhancement and hearing loss.
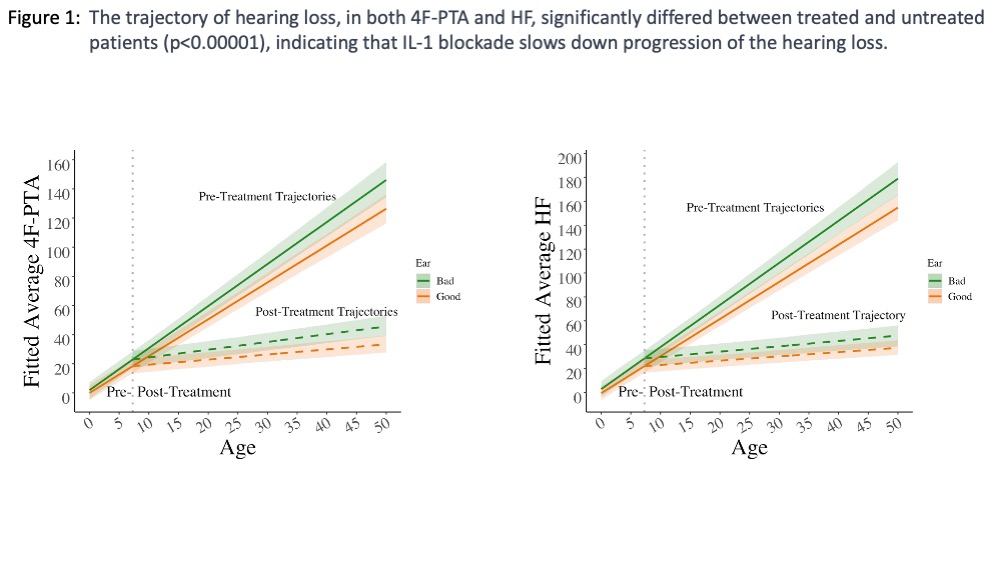
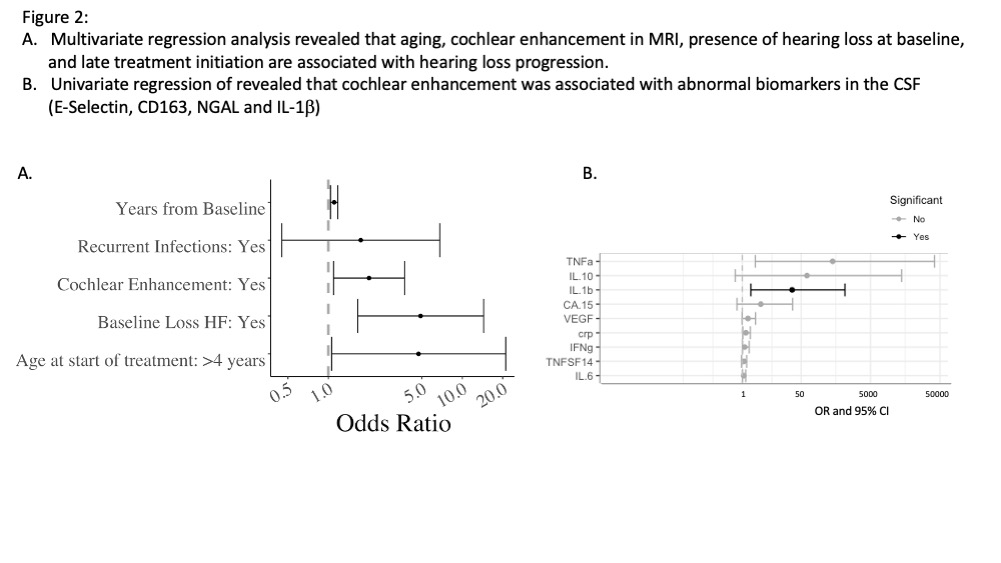
Results: The trajectory of hearing loss significantly differed between treated and untreated patients, indicating that IL-1 blockade slows down progression (p< 0.00001) (Figure 1). Yearly 4F-PTA increase on treatment was 0.35 dB in the good ear and 0.5 dB in the bad ear, compared to a 2.5 dB increase in the good ear and 2.9 dB in the bad ear prior to treatment. Yearly HF hearing loss worsened by 0.36 dB in the good ear (0.5 dB bad ear), compared to 3.11 dB in the good ear and 3.5 dB in the bad ear prior to treatment (Figure 1). Cochlear enhancement, age at start of treatment and baseline hearing loss significantly contributed to hearing loss progression; recurrent infections did not reach significance. The univariate logistic regression identified elevated cytokines associated with cochlear enhancement in the corresponding MRI. In 36 pts’ CSF, IL-1b, and markers of endothelial, neutrophil and macrophage activation, E-Selectin, NGAL, and CD163, were significantly associated with cochlear enhancement.
Conclusion: Hearing loss continues to progress more than expected for age despite treatment. IL-1 blocking treatment (84 % on anakinra) in NOMID patients however significantly retards progression of hearing loss. Baseline hearing loss, older age at the start of treatment, and cochlear enhancement on brain MRI were associated with faster progression of hearing loss in treated NOMID patients. Cochlear enhancement was significantly associated with CSF levels of IL-1b and markers of neutrophil and macrophage activation, suggestive of subclinical inflammation as driver of onging hearing loss.
To cite this abstract in AMA style:
Alehashemi S, Ortega-Villa A, Garg M, Myint-Hpu K, Johnson K, BHUYAN F, King K, Zalewski C, Fink D, Kuhns D, Butman J, Brewer C, Kim H, Follmann D, Goldbach-Mansky R. IL-1 Blocking Treatment Slows the Progression of Sensorineural Hearing Loss in Patients with NOMID [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/il-1-blocking-treatment-slows-the-progression-of-sensorineural-hearing-loss-in-patients-with-nomid/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/il-1-blocking-treatment-slows-the-progression-of-sensorineural-hearing-loss-in-patients-with-nomid/