Session Information
Date: Tuesday, November 7, 2017
Title: Pediatric Rheumatology – Clinical and Therapeutic Aspects Poster III: Juvenile Arthritis
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The efficacy and safety of intravenous (IV) tocilizumab (TCZ), an interleukin-6 receptor-alpha inhibitor, have been demonstrated in patients with polyarticular-course juvenile idiopathic arthritis (pcJIA) (Brunner HI et al. Ann Rheum Dis. 2014;74:1110-7). This study investigated appropriate dosing regimens of subcutaneous (SC) TCZ in a similar population.
Methods: We enrolled patients aged 1-17 years with pcJIA who had inadequate response/intolerance to methotrexate and were TCZ naive or received TCZ IV with adequate disease control. TCZ SC was administered open label according to a body weight (BW)–based dosing regimen based on pharmacokinetic (PK) simulations aimed at obtaining TCZ trough exposures at or above those achieved by approved IV dosing in pcJIA patients. pcJIA patients weighing <30 kg received TCZ 162 mg every 3 weeks (Q3W) while those weighing ≥30 kg received TCZ 162 mg Q2W for 52 weeks. Adequacy of TCZ dosing was assessed by comparing model-estimated PK parameters (Ctrough, Cmax, area under the concentration-time curve) with the IV exposure data; safety and efficacy (exploratory; JADAS71) were also measured.
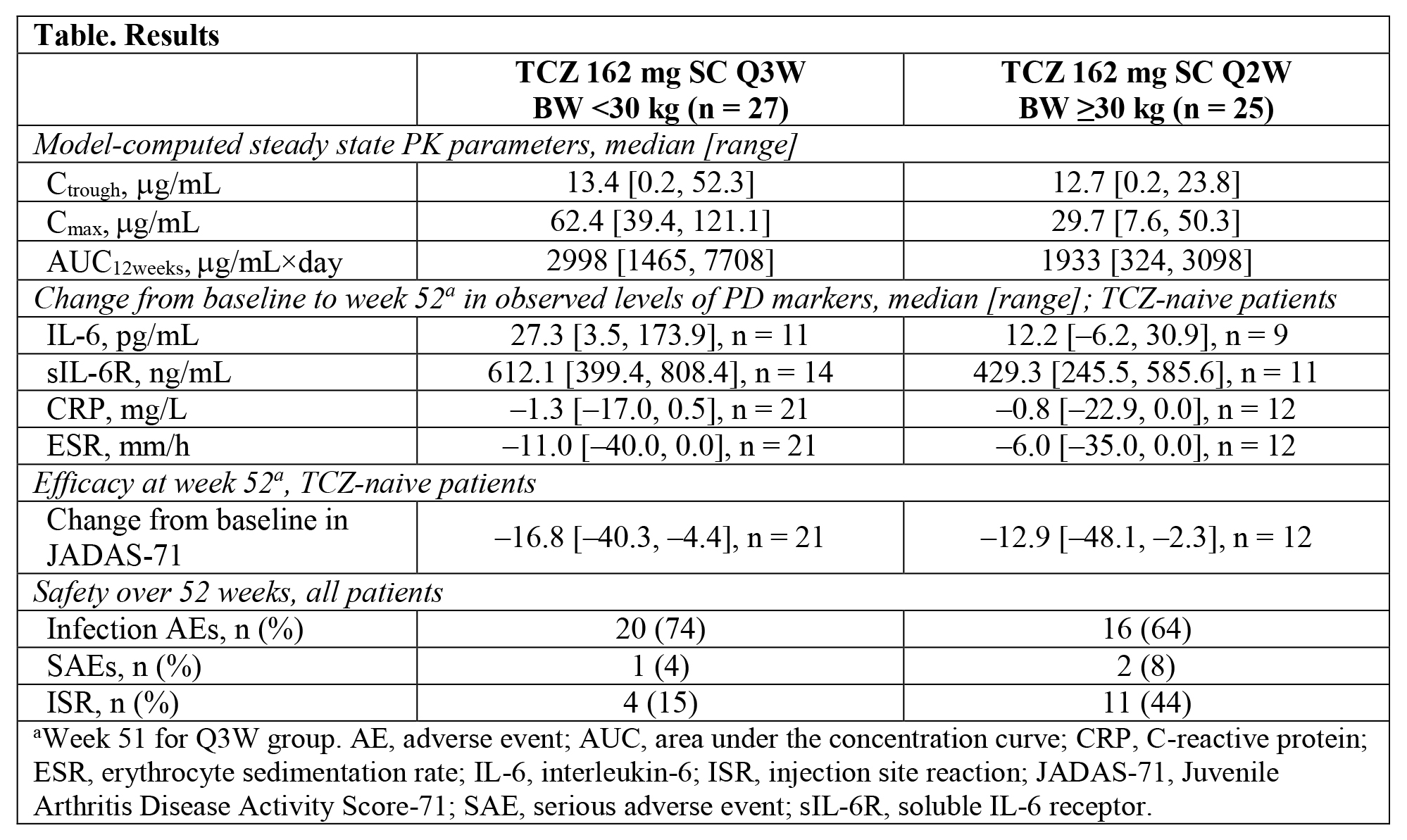
Results: Among the 52 patients (69% female) enrolled, 27 patients had <30 kg BW and 25 patients ≥30 kg BW. Median baseline age was 6.0 years for the <30 kg BW group and 15.0 years for the ≥30 kg BW group. In the <30 kg BW group 85% were TCZ naive, and in the ≥30 kg BW group 56% were TCZ naive. Given similarities in steady state PK of the naive vs non-naive patients, pooled data analyses are presented. Median Ctrough was similar between BW groups and higher than with TCZ IV in both BW categories, confirming adequate exposure from SC doses. Cmax and AUC12weeks were higher in the <30 kg BW than the ≥30 kg BW TCZ SC group. Consistent with SC vs IV administration, median Cmax from SC dosing was lower than with IV dosing in both BW groups. Changes in PD parameters for TCZ-naive patients were consistent with those previously observed for TCZ IV, suggesting adequate reduction of inflammation. JIA activity (JADAS-71) improved in both BW groups (Table), with trends consistent with those observed for TCZ IV. Infections were the most frequent adverse event (AE). Four serious AEs occurred in 3 patients (croup and varicella [same patient], anorexia, and arthralgia). Injection site reactions were more common in the ≥30 kg BW group (Table). No serious hypersensitivity, AE leading to withdrawal, opportunistic infection, serious hepatic AE, or death occurred. The overall rate of AEs was 7.9/100 patient-years, consistent with that for TCZ IV in pcJIA.
Conclusion: The BW-based TCZ SC dosing regimens for pcJIA provided adequate exposure to support efficacy comparable to that of TCZ IV, with an acceptable benefit-risk profile.
To cite this abstract in AMA style:
Brunner HI, Ruperto N, Martini A, Ramanan AV, Cuttica R, Weiss JE, Henrickson M, Schmeling H, Anton J, Minden K, Hsu J, Bharucha K, Wimalasundera S, Kadva AK, Upmanyu R, Mallalieu NL, Douglass W, Lovell DJ, De Benedetti F. Identification of Optimal Subcutaneous Doses of Tocilizumab in Children with Polyarticular-Course Juvenile Idiopathic Arthritis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/identification-of-optimal-subcutaneous-doses-of-tocilizumab-in-children-with-polyarticular-course-juvenile-idiopathic-arthritis-2/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/identification-of-optimal-subcutaneous-doses-of-tocilizumab-in-children-with-polyarticular-course-juvenile-idiopathic-arthritis-2/