Session Information
Date: Saturday, November 7, 2020
Title: SLE – Treatment Poster I
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: 2016 ophthalmology guidelines recommend using hydroxychloroquine (HCQ) dosages < 5mg/kg/day, which is lower than the traditional 400mg/day for the majority of SLE patients. However, it is unknown whether lower dosages retain efficacy for controlling SLE disease activity. The aim of this study was to determine trends of HCQ dose reduction among SLE patients and identify a potential association with the risk of lupus flares.
Methods: We identified a cohort of patients with SLE from our academic medical center using a previously validated electronic health record SLE phenotype algorithm (PPV 92%) (Jorge A et al. Semin Arthritis Rheum 2019) and included patients who met 1997 ACR classification criteria and who had at least two visits in our academic rheumatology practice since 1/1/2016. We excluded patients who did not use HCQ at any time after that date. We obtained demographics, anthropometrics, medication use, SLE disease history, and SLE manifestations and disease activity assessed at each rheumatology visit. HCQ dose was assessed as a time-varying exposure, in mg/day as 400, 200, or between 200-400 (typically 300). We identified all dose change events. The primary outcomes were combined mild, moderate, and severe SLE flares defined by the revised SELENA-SLEDAI flare index (rSSFI). The secondary outcome was severe rSSFI flares alone. We determined the rates of SLE flares overall, prior to HCQ dose changes, and following HCQ dose reduction and calculated SLE flare incidence rate ratios.
Results: 216 patients met the inclusion criteria, with a mean 2.6 years of follow up since 2016. Patients were on average 46.2 years of age and were 90.3% female (Table 1). A total of 111 patients (51.4%) were treated with a HCQ dose of 400mg/day without a dose change, whereas 33 patients (15.3%) received 200mg/day and 12 (5.6%) received 300mg/day without a dose change during the study period. 30 patients (13.9%) initially received 400mg/day and underwent HCQ dose reduction (n=18 to 200mg/day, n=12 to 300mg/day). A higher proportion of patients who underwent dose reduction had body weight under 80kg (thus 400mg of HCQ would exceed 5mg/kg), but the dose also exceeded 5mg/kg for 51.7% of patients who remained on 400mg/day throughout study follow up. The overall rSSFI flare rates were 0.39 total flares per person-year (PY) and 0.06 severe flares per PY. For patients who received 400mg/day throughout study follow up, the overall flare rate and severe flare rate were 0.48 per PY and 0.08 per PY, respectively. Among patients who underwent HCQ dose reduction, overall SLE flare rates were 0.14 per PY prior to HCQ dose reduction from 400mg to 200mg/day and 0.30 per PY following dose reduction to 200mg. The SLE flare rates were 0.13 per PY prior to HCQ dose reduction from 400mg to 300mg/day and 0.24 per PY following dose reduction to 300mg (Table 2).
Conclusion: In recent years, a minority of patients with SLE underwent HCQ dose reduction. Of those who did, we found a numerically higher incidence of SLE flares following HCQ dose reduction. This finding should be replicated in a larger cohort to clarify a potential loss of benefits associated with lowering the treatment dose.
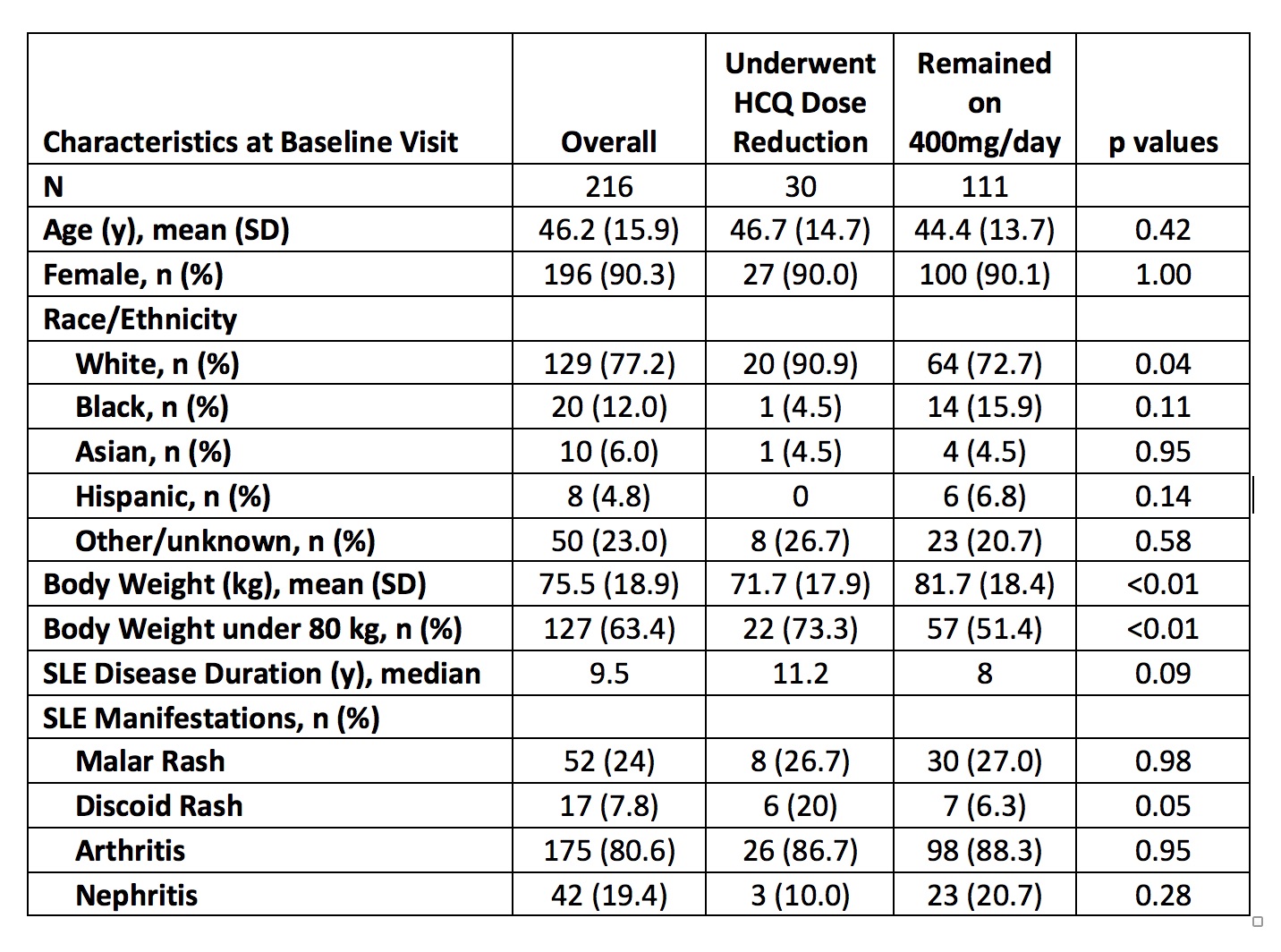 Table 1. Baseline Characteristics of SLE Patients According to Time-Varying Hydroxychloroquine Dose
Table 1. Baseline Characteristics of SLE Patients According to Time-Varying Hydroxychloroquine Dose
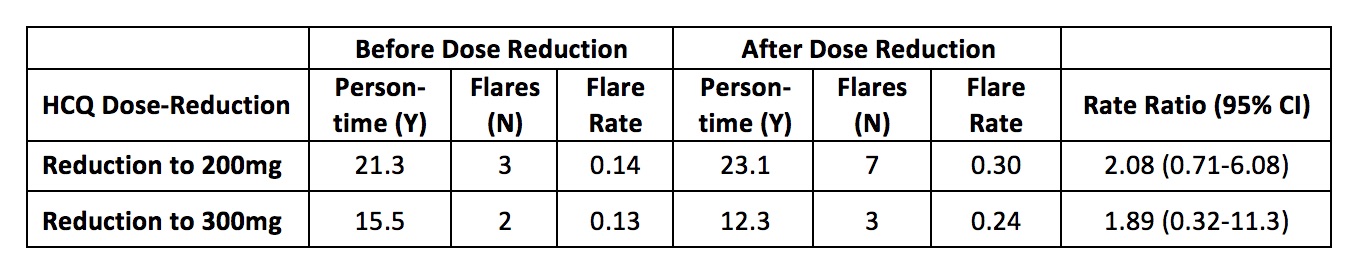 Table 2. Hydroxychloroquine Dose-Reduction and the Incidence of SLE Flares
Table 2. Hydroxychloroquine Dose-Reduction and the Incidence of SLE Flares
To cite this abstract in AMA style:
Jorge A, Ho G, Wu M, Costenbader K, Choi H. Hydroxychloroquine Dose Reduction and SLE Flares [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/hydroxychloroquine-dose-reduction-and-sle-flares/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-dose-reduction-and-sle-flares/
