Session Information
Date: Monday, November 8, 2021
Title: Abstracts: SLE – Treatment: New Agents, Old Agents (1458–1463)
Session Type: Abstract Session
Session Time: 4:30PM-4:45PM
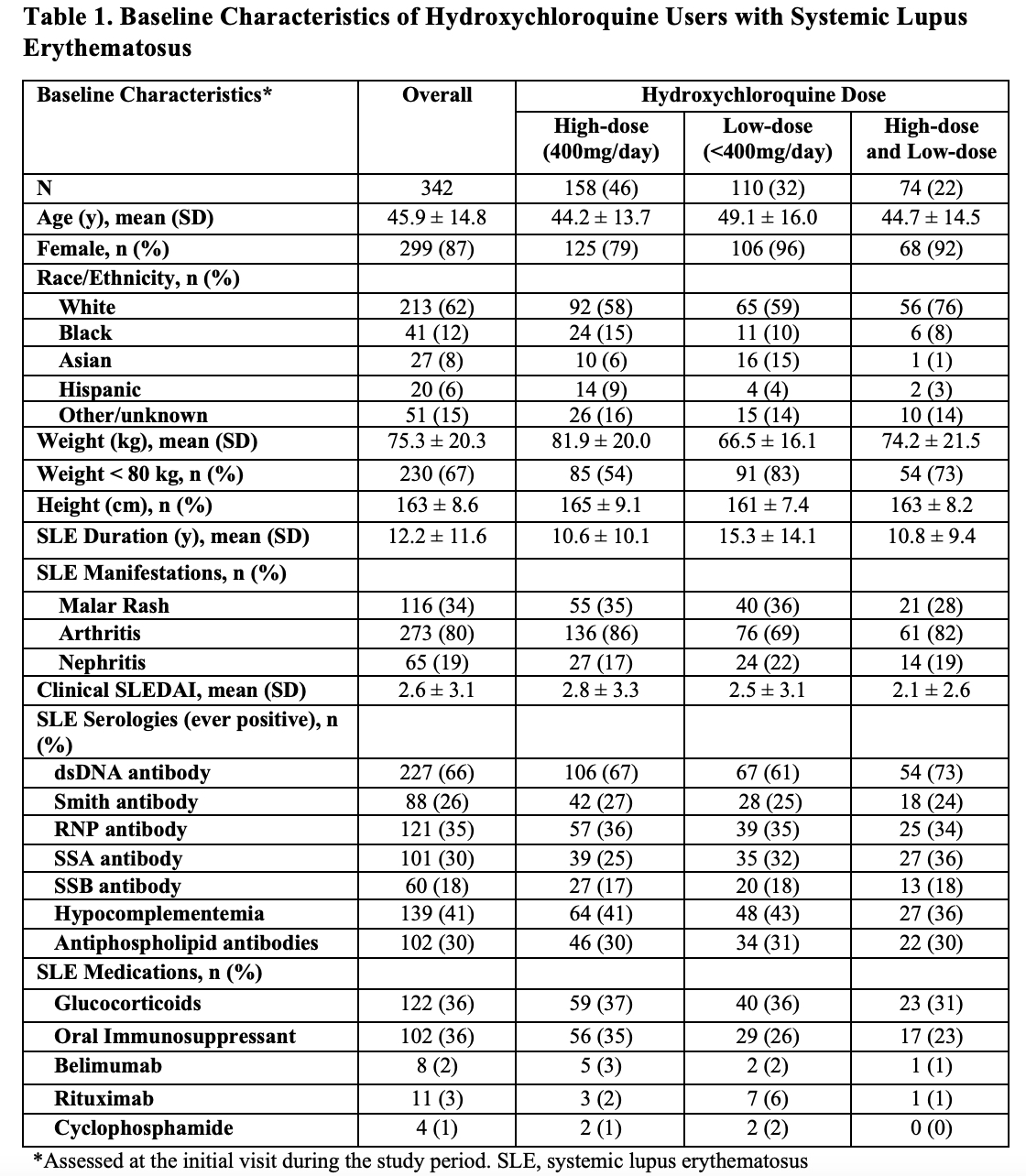
Background/Purpose: Hydroxychloroquine (HCQ) is an important treatment for systemic lupus erythematosus (SLE), known to reduce disease activity and flares. To minimize the risk of toxicity, recent guidelines recommend using HCQ dosages less than 5mg/kg/day, which is less than 400mg/day for many patients. However, the dose needed to prevent lupus flares is unknown. The aim of this study was to determine the association between HCQ dose and the risk of lupus flares.
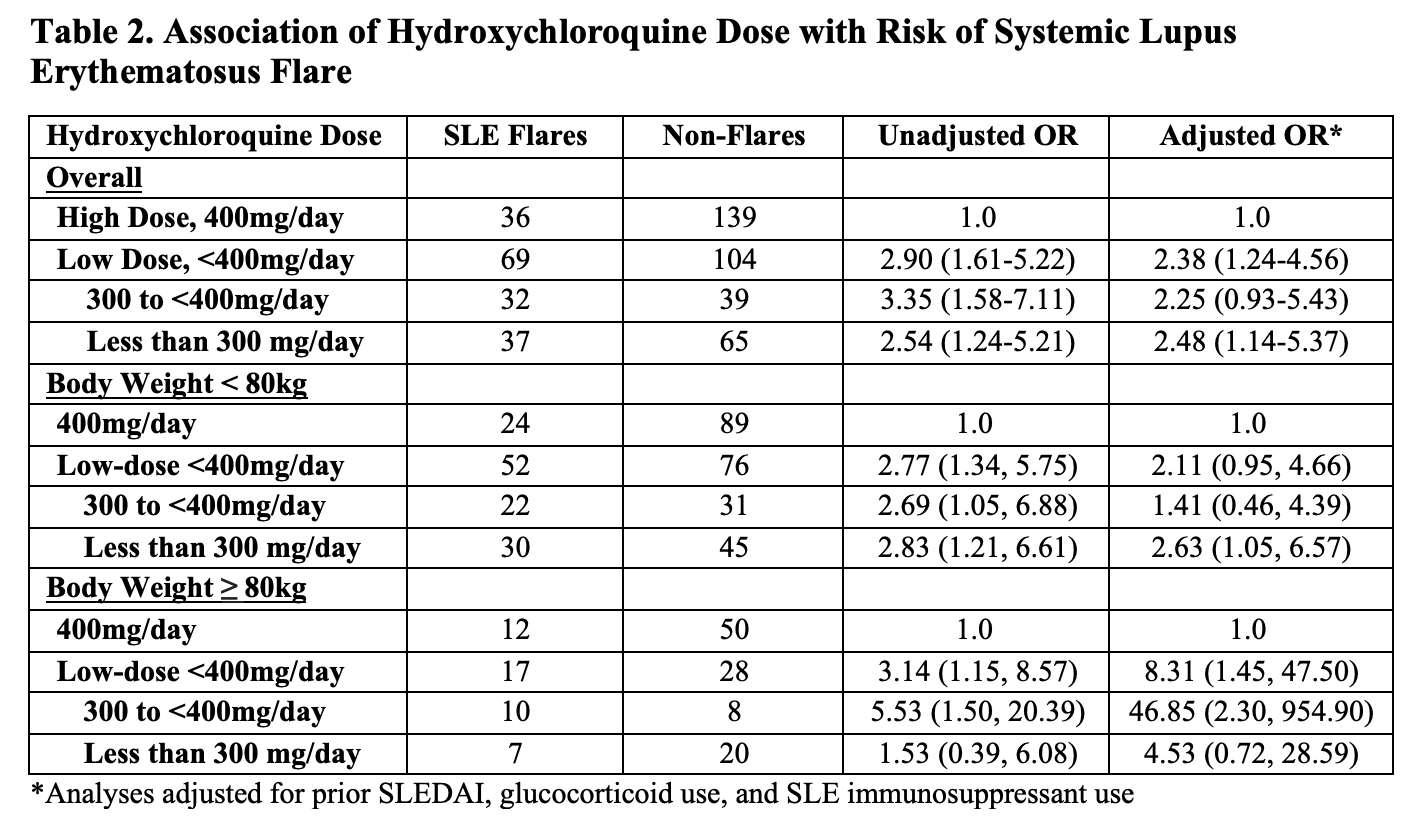
Methods: We identified a cohort of patients with SLE taking HCQ who met 1997 ACR classification criteria and had at least two rheumatology visits between January 2016 and December 2020. We obtained demographics, anthropometrics, medications, SLE history and disease manifestations, and disease activity (e.g., clinical SLEDAI) assessed at each rheumatology visit. The outcome of interest was SLE flare defined by the revised SELENA-SLEDAI flare index (rSSFI). Among patients who had at least one SLE flare during the study period, we performed a case-crossover study. Each patient’s follow up time was divided into case periods, defined by the 6-months prior to each flare, as well as control periods, defined as 6-month periods with no SLE flares (Figure 1). The exposure of interest was HCQ dose, categorized as high-dose (e.g. 400 mg/day) or low-dose (e.g., < 400 mg/day); low-dose was further categorized into < 300 and between 300 and < 400mg. The average HCQ dose was calculated for each case and control period. Conditional logistic regression was used to estimate the odds ratios (ORs) and 95% confidence intervals (CI)s for SLE flare associated with low-dose HCQ use compared with high-dose, and we stratified patients according to body weight. We adjusted for time-varying glucocorticoid use, SLE immunosuppressant use, and clinical SLEDAI score prior to each six-month observation period.
Results: Of 342 patients with SLE who used HCQ, mean age was 46 years, and 87% were female (Table 1). Body weight was below 80kg for 67% of patients, thus 400mg would exceed 5mg/kg/day. 74 patients (22%) received high-dose and low-dose HCQ during the study period, whereas 158 (46%) and 110 (32%) received only high-dose and only low-dose HCQ, respectively. There were 466 total rSSFI SLE flares over 2,176 visits during the study period, including 105 flares among those who received both high-dose and low-dose HCQ. The adjusted OR for SLE flare associated with low-dose HCQ was 2.38 (95% CI 1.24-4.56) (Table 2). For patients weighing less than 80kg/day, a HCQ dose under 300mg/day was associated with an increased risk of SLE flares. For patients weighing at least 80kg, any HCQ dose under 400mg/day was associated with an increased risk of SLE flares.
Conclusion: Low-dose HCQ use was associated with an increased risk of SLE flares. The risks of toxicity and benefits for preventing SLE flares must be balanced in choosing the optimal HCQ dose.
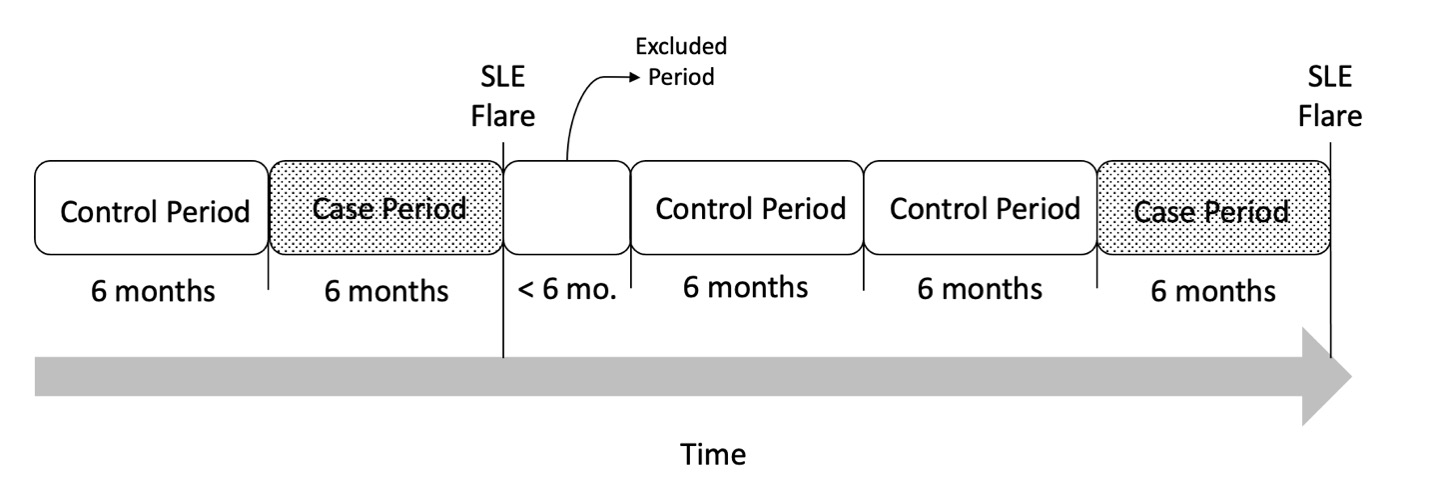 FIGURE 1. Case-crossover study design. Each patient’s follow up time was divided into case periods defined by the 6-months prior to each flare as well as control periods defined as 6-months periods without a SLE flare. Each patient could have multiple case and/or control periods, and control periods could occur before or after case periods. Additional periods shorter than 6 months were excluded from the analysis.
FIGURE 1. Case-crossover study design. Each patient’s follow up time was divided into case periods defined by the 6-months prior to each flare as well as control periods defined as 6-months periods without a SLE flare. Each patient could have multiple case and/or control periods, and control periods could occur before or after case periods. Additional periods shorter than 6 months were excluded from the analysis.
To cite this abstract in AMA style:
Jorge A, Mancini C, Fu X, Ho G, Zhang Y, Costenbader K, Choi H. Hydroxychloroquine Dose and the Risk of Systemic Lupus Erythematosus Flares [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/hydroxychloroquine-dose-and-the-risk-of-systemic-lupus-erythematosus-flares/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-dose-and-the-risk-of-systemic-lupus-erythematosus-flares/