Session Information
Date: Sunday, November 12, 2023
Title: (0460–0479) Reproductive Issues in Rheumatic Disorders Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
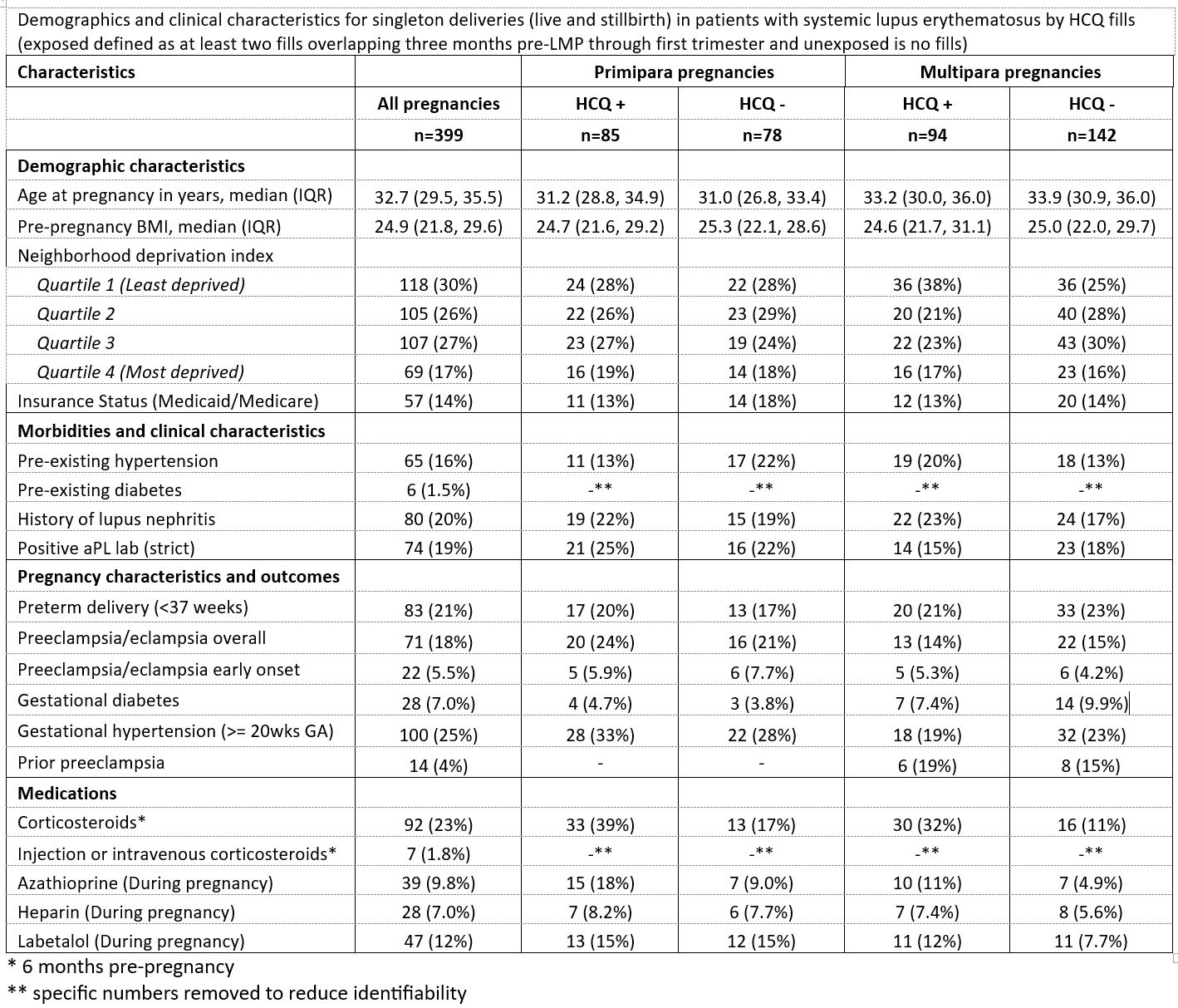
Background/Purpose: Pregnancies in patients with systemic lupus erythematosus (SLE) are at greater risk of preeclampsia. Hydroxychloroquine (HCQ) is recommended during SLE pregnancy to control disease activity. Some studies have suggested that HCQ may reduce the risk of preeclampsia and hypertensive disorders of pregnancy, although results are conflicting. We studied HCQ use in SLE pregnancy in a large integrated health system to investigate whether HCQ reduces the risk of preeclampsia in a diverse patient cohort.
Methods: SLE was defined as ≥2 coded visits ≥7 days apart (ICD9: 710.0, ICD10: M32.1, M32.9). Individuals with full pregnancy histories derived from the electronic health records occurring in 2011-2020 were included. Data from all healthcare encounters, labs and pharmacy records were accessible. Qualifying SLE-coded encounters were required to occur before last menstrual period date (LMP) prior to pregnancy. We included pregnancies lasting until at least 20 gestational weeks. Pregnancies were considered HCQ-exposed if at least two fills covered the exposure window (3 months pre-LMP through 1st trimester), and unexposed if there were no fills overlapping with this time. Preeclampsia and eclampsia (PE/E) were defined in the pregnancy database and included all ICD-coded visits during pregnancy and labor and delivery documentation. All analyses were stratified by parity (primipara vs multipara). To account for possible confounding by indication, we estimated propensity scores (PS) as a function of age, BMI, race/ethnicity, neighborhood deprivation index, pre-pregnancy morbidity (diabetes, hypertension, nephritis) and medication ((corticosteroids, azathioprine), and antiphospholipid antibody (aPL) status. We estimated risk ratios (RR) and 95% confidence intervals using PS adjusted Poisson regression stratified by parity and by pre-pregnancy hypertension to investigate effect modification.
Results: 18% of SLE pregnancies had a PE/E diagnosis; among primiparous: 24% in HCQ exposed vs 21% in unexposed, and 14% and 15% among multiparous exposed and unexposed, respectively. Approximately 20% of the pregnancies had ≥1 aPL antibody, 16% had pre-existing hypertension, and 20% had a history of nephritis in the study cohort of 399 SLE pregnancies. (Table 1). In PS-adjusted models among primipara we found a null association (1.06, 95% CI: (0.57, 1.97)). Among multipara the RR was 0.76 (0.35, 1.64). Examining effect modification by pre-pregnancy hypertension, we found that among those with pre-pregnancy hypertension, ≥2 HCQ fills (vs no fills) was associated with a RR of 0.70 (95% CI (0.39, 1.25)), compared to RR=1.12 (0.57, 2.20) among those without pre-existing hypertension.
Conclusion: SLE patients using HCQ shortly before and early in pregnancy generally were comparable to those who did not use HCQ, although some small differences were observed. Confounding by disease activity was accounted for by including pre-pregnancy treatment with corticosteroids and azathioprine. We consistently observed RRs near or below the null of 1.0.
To cite this abstract in AMA style:
Simard J, Liu E, Rector A, Cantu M, Kuo D, Chakravarty E, Druzin M, Shaw G, Weisman M, Hedderson M. Hydroxychloroquine and Preeclampsia Risk in Lupus Pregnancy: Results from a Large Regional Integrated Health Network [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/hydroxychloroquine-and-preeclampsia-risk-in-lupus-pregnancy-results-from-a-large-regional-integrated-health-network/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hydroxychloroquine-and-preeclampsia-risk-in-lupus-pregnancy-results-from-a-large-regional-integrated-health-network/