Session Information
Date: Sunday, November 8, 2015
Title: Osteoarthritis - Clinical Aspects Poster I: Treatments and Metabolic Risk Factors
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Although only few nonoperative treatment options for knee
osteoarthritis (OA) are available,
there continues to be debate about the effectiveness of hyaluronic acid (HA)
injections, particularly in light of recent clinical practice guidelines by
AAOS and recommendations by OARSI, ACR, and recent systematic reviews. Thus, we investigated whether the formulation of
HA may be contributing to some of the reported variation in clinical outcomes. This study evaluated whether the use of HA
injections is associated with a delay to knee arthroplasty
(KA) and whether there was a difference in effect size by formulation of
HA injections.
of Part B Medicare data from 2007 to 2012 was used to
identify knee OA patients who underwent KA. The time to KA was compared between
patients who were coded with HA injection use (“HA”) and those who were not
(“no HA”), using quantile regression with propensity score adjustment. These were
further stratified by HA formulation between bioengineered HA (high
molecular weight (HMW) and medium MW (MMW) Bio-HA) and non-bioengineered HA (high
MW (HMW) and low MW (LMW) non-Bio-HA).
Results: A total of 23,008 knee OA patients (n=17,007
“no HA”; n=6,001 “HA”) subsequently underwent KA and were included in the study.
After adjusting for potential confounding patient factors and propensity scores,
the “HA” cohort was found to be associated with a delay to KA of 7.1 months
(95% CI: 6.6 to 7.5 months; p<0.001) compared to the “no HA” cohort. When
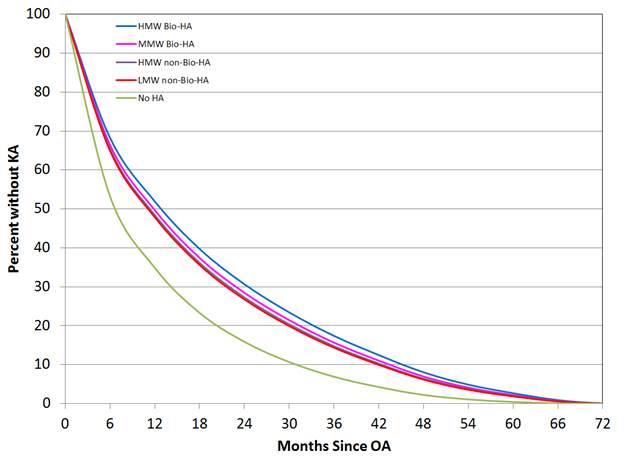
compared to the “no HA” cohort, patients who were treated with HMW Bio-HA were
found to have the longest time to KA over patients treated with other HA
formulations (Figure 1 and Table 1). However, when comparing between HA
formulations, LMW non-Bio-HA was found to have the shortest time to KA, while the
remaining three formulations had similarly longer time to KA (Table 1).
Figure 1: Survivorship curves (KA as the endpoint) for “no
analysis of elderly knee OA patients showed a
significantly longer delay to KA for those who were treated with HA. The delay
may provide additional time for patients to better control pre-existing
conditions prior to KA, which could aid in reducing postoperative morbidity.
Furthermore, there appears to be some differences in the effect of HA
formulation, in particular molecular weight, on the delay to KA.
HA” and “HA” cohorts.
KA (in months) between patient cohorts with propensity score adjustment (*positive
means more delay, negative means less delay)
To cite this abstract in AMA style:
Ong K, Anderson A, Lau E, Niazi F, Fierlinger A, Kurtz S, Altman R. Hyaluronic Acid Injections in Knee Osteoarthritis Patients Are Associated with Delay to Knee Arthroplasty [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/hyaluronic-acid-injections-in-knee-osteoarthritis-patients-are-associated-with-delay-to-knee-arthroplasty/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/hyaluronic-acid-injections-in-knee-osteoarthritis-patients-are-associated-with-delay-to-knee-arthroplasty/