Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: BNT162b2 is the most commonly used COVID-19 vaccine in Japan. The safety and efficacy of the vaccine was demonstrated in the general population, but patients receiving immunosuppressive (IS) therapy were excluded from the study. Recently, we have demonstrated and reported that immunogenicity to BNT162b2 is decreased in Japanese rheumatoid and musculoskeletal disease (RMD) patients under MTX treatment similar to Western reports. However, the differential effects of various IS drugs including MTX on the immunogenicity of this vaccine have not yet been fully elucidated. Therefore, we examined the effect of each IS agent on SARS-CoV-2 spike protein antibody production after BNT162b2 vaccination in Japanese RMD patients undergoing various types of treatment.
Methods: One hundred and ninety-eight outpatients with RMD consisting of 79 RA and 119 non-RA patients at Kagawa University Hospital and 43 healthy volunteers were included in the study. All participants had received two doses of BNT162b2 between March 2021 and September 2021. Serum sample was collected at least 14 days after the second dose. Antibody titre against SARS-CoV-2 spike protein in serum was measured by ELISA (Elecsys Anti-SARS-CoV-2 S RUO). The antibody titer and antibody positive rate were analyzed. An antibody titre of 15U/mL or more was considered positive. We analyzed the relationship between treatment of RMD and humoral immune response. Statistical analysis was performed using JMP® Pro 14 (SAS Institute, Cary, NC, USA).
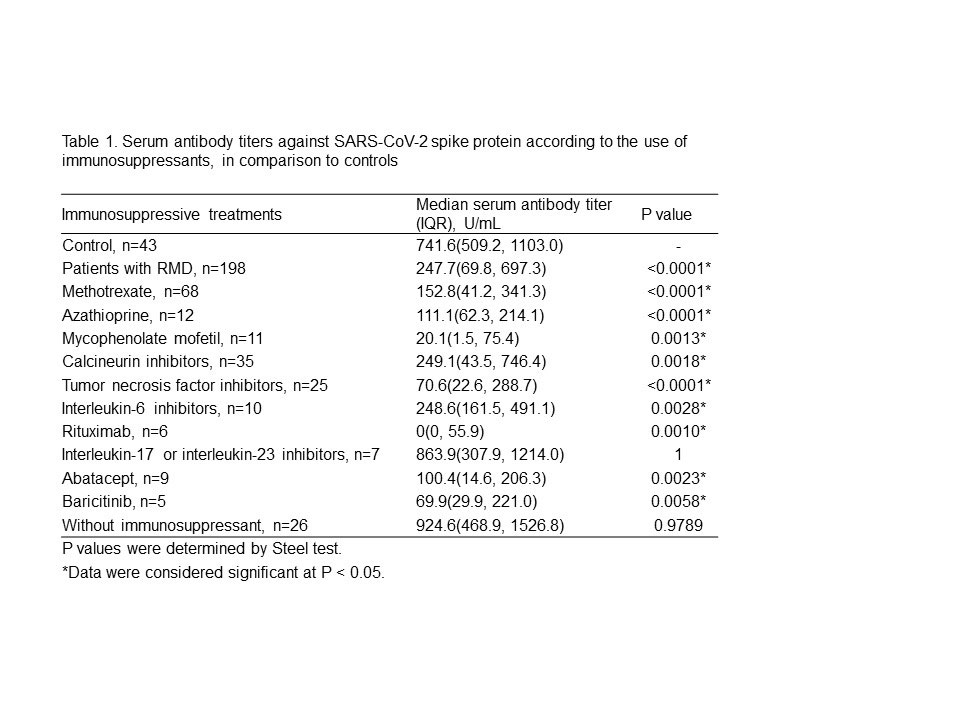
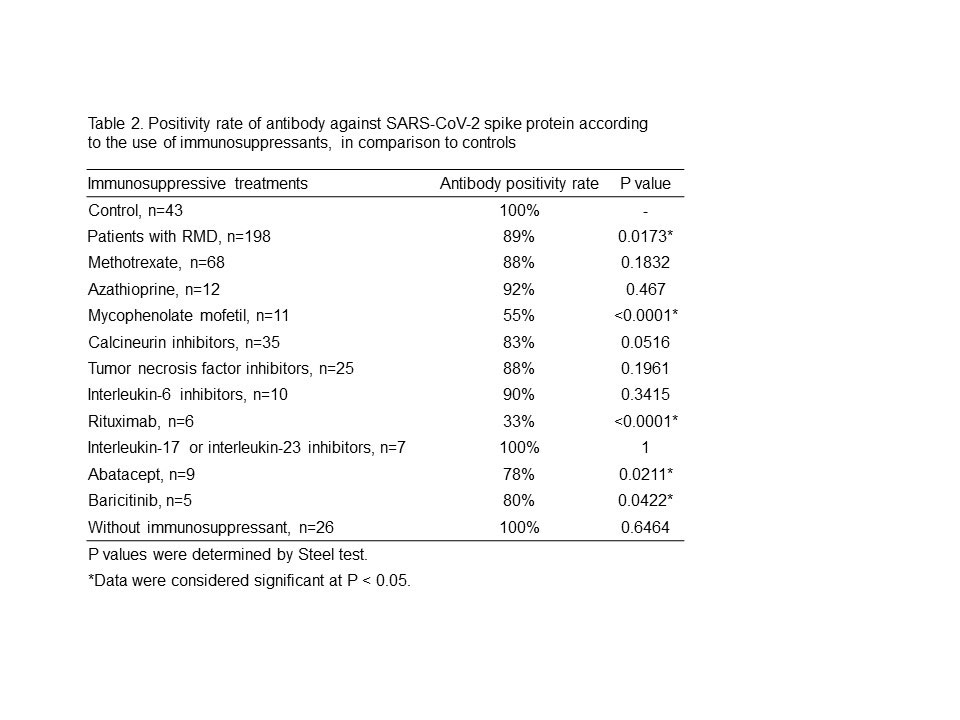
Results: Table 1 shows the analysis of antibody titers. RMD patients had significantly lower antibody titers than controls. However, RMD patients not treated with IS showed no difference in antibody titers compared to controls. Among the treatments, antibody titers in RMD patients treated with rituximab (RTX) were markedly lower than those in controls. IL-17 and IL-23 inhibitors did not impair the humoral response. Other IS drugs were found to have modestly lower antibody titers. Table 2 shows the analysis of antibody positivity rate. Antibody positivity rates in all RMD patients were also significantly lower than in controls. MMF, RTX, abatacept (ABT) and baricitinib (Bari)-treated patients had significantly lower antibody positive rates than controls, but MTX-treated patients did not show a significant difference. There were no significant differences in antibody positivity rate between MTX monotherapy patients and those receiving MTX with TNF inhibitors (TNFi) or IL-6 inhibitors (IL-6i). There was also no difference between TNFi and IL-6i treatment among these patients.
Conclusion: Most IS drugs decreased immunogenicity against BNT162b2 in Japanese RMD patients. The degree of decrease in antibody titer differed among IS drugs. MMF, RTX, Bari and ABT decreased both antibody titer and antibody positivity rate. MTX decreased antibody titer but had no significant effect on antibody positivity rate. There was no effect of concomitant biologics with MTX on antibody positivity rate. The antibody titer required to prevent COVID-19 infection and progression to severe disease has not yet been determined. The search for effective antibody cutoff value induced by BNT162b2 is warranted for safe and effective treatment of RMD.
To cite this abstract in AMA style:
Sugihara K, Wakiya R, Kameda T, Shimada H, nakashima S, Kato M, Miyagi T, mizusaki m, Mino R, Dobashi H. Humoral Immune Response Against BNT162b2 mRNA COVID-19 Vaccine in Patients with Rheumatic Disease Undergoing Immunosuppressive Therapy: A Japanese Monocentric Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/humoral-immune-response-against-bnt162b2-mrna-covid-19-vaccine-in-patients-with-rheumatic-disease-undergoing-immunosuppressive-therapy-a-japanese-monocentric-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/humoral-immune-response-against-bnt162b2-mrna-covid-19-vaccine-in-patients-with-rheumatic-disease-undergoing-immunosuppressive-therapy-a-japanese-monocentric-study/