Background/Purpose: The subsets of primary Sjögren’s syndrome (pSS) patients justifying biological therapy (BT) remain a matter of debate. Our goal was [1] to describe which inclusion criteria have been used in all previous studies using BT in pSS and [2] to evaluate the proportion of patients who may be included in further trials evaluating a biologic according to the chosen criteria.
Methods: We performed a literature review using pubmed and clinical-trial.gov of all studies evaluating BT in pSS to identify their inclusion criteria. Then, we evaluated in the ASSESS cohort (a French national multi-center prospective cohort set up in 2006 to identify valuable predictive factors lymphoma during a 5-year prospective follow-up in which 15 tertiary centers included 410 patients with pSS) the proportion of pSS patients who could be included in a trial according to the chosen criteria.
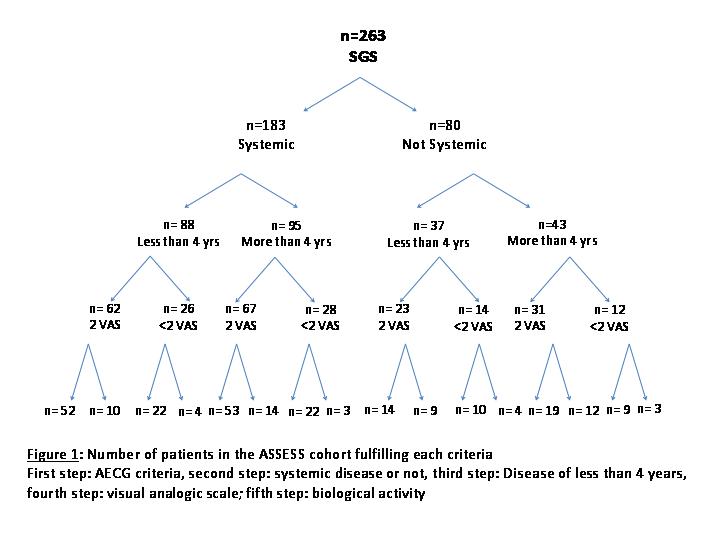
Results: Our literature review identified 16 studies evaluating biologics in pSS: Two open label studies and two double blind studies have been conducted to evaluate anti TNF. Only one open label study evaluated epratuzumab, in patients with B-cell activity. Five open-label studies and 4 randomized studies evaluated or are evaluating prospectively rituximab. Two studies are ongoing with belimumab. The main frequently used inclusion criteria were AECG criteria, disease duration (less than 4, 5 or 10 years), presence of systemic involvement (ESSDAI>1), visual analogic score (VAS) for dryness, pain, and fatigue higher to 5/10 and biological markers of activity (hypergammaglobulinemia and/or cryoglobulinemia and/or β2 microglobulinemia and/or low level of C4). In the ASSESS cohort, 263 patients had all data available. Table 1shows a tree representing the number of patients of the ASSESS cohort which fulfil each of these criteria. Their combinations show that if we limit inclusion to patients with systemic and recent pSS (less than 4 years), with 2/3 VAS>5/10 and biological activity, only 52/263 (20%) of the patients could be included. If we consider systemic or recent pSS, 2/3 VAS>5/10 and biological activity, 166/263 (63%) could be included.
Conclusion: The number of patients that could be include in a trial evaluating pSS, depends highly on the inclusion criteria. Even with a slightly selective criterion of systemic involvement (ESSDAI >1), a small number of patients with very recent disease could be included. Most patients reported 2 VAS higher to 50/100 and have biological markers of activity.
Disclosure:
V. Devauchelle-Pensec,
None;
X. Mariette,
None;
J. E. Gottenberg,
None;
R. Seror,
None;
A. L. Fauchais,
None;
O. Vittecoq,
None;
V. Le Guern,
None;
J. Morel,
Roche Pharmaceuticals,
5;
J. Dubost,
None;
P. Dieude,
None;
E. Hachulla,
None;
P. Y. Hatron,
None;
C. Larroche,
None;
A. Perdriger,
None;
X. Puechal,
Pfizer Inc,
5,
Roche Pharmaceuticals,
5;
D. Sene Sr.,
None;
S. Rist,
None;
A. Saraux,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/how-to-better-define-inclusion-criteria-in-a-large-controlled-trial-in-primary-sjogren-syndrome/