Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Uptake of biosimilars has been suboptimal in North America. In 2019, British Columbia (BC) became the first jurisdiction in North America to require patients with inflammatory arthritis to switch to a biosimilar to maintain coverage. This study quantifies the impact of this policy intervention— and a previous policy that required new initiators to a biologic to use a biosimilar — on uptake and spending on biosimilar infliximab and etanercept in British Columbia (BC).
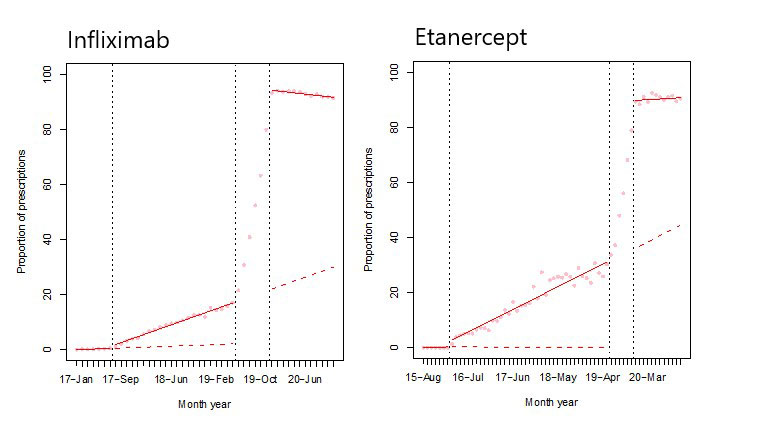
Methods: We conducted a quasi-experimental interrupted time series analysis using administrative claims data in BC from January 2013 through November 2020. Two policy interventions were considered: (1) new starts, whereby individuals initiating infliximab or etanercept for the first time were required to start the biosimilar (implemented in Feb 2016 and July 2017 respectively), and (2) mandatory switching, which required those already receiving infliximab or etanercept to switch to the biosimilar version in order to maintain coverage (implemented between May 2019 and Nov 2019). Large commercial insurers which provide supplementary drug coverage also introduced biosimilars policies in line with the BC provincial government. Under both biosimilar switching policies, exceptional coverage to reference products was allowed if medically needed.
A segmented linear regression was used to model the level and trend change in biosimilar etanercept and infliximab utilization and spending before and after the two policy interventions.
Results: We identified 208,984 BC residents ≥18 years with rheumatoid arthritis, ankylosing spondylitis, and psoriatic arthritis who qualified for public drug coverage. After the new start policy, we detected a small gradual increase in the proportion of biosimilar etanercept prescriptions dispensed of 0.65% (95%CI 0.44, 0.85) per month. The monthly trend related to the proportion of total spending on biosimilar etanercept also increased (0.51, 95%CI 0.28, 0.73). After the mandatory switching policy, there was a sustained increase in the proportion of biosimilar etanercept and infliximab prescriptions dispensed of 76.98% (95%CI 75.56, 78.41) and 58.43% (95%CI 52.11, 64.75), respectively. Similarly, there was a persistent increase in spending on biosimilar etanercept and infliximab of 78.22% (95%CI 76.65, 79.79) and 71.23% (95%CI 66.82, 75.65), respectively.
Conclusion: Our study found new start policies resulted in only small, gradual increases in biosimilar utilization. A mandatory switch policy had a marked immediate and sustained impact on uptake. Further analysis will examine changes in other health utilization, and long-term impact on persistence on biosimilar treatment. These findings are particularly relevant to areas with a more concentrated insurance system where mandatory switching policies could lead to greater savings.
To cite this abstract in AMA style:
McClean A, Bansback N, Cheng L, Clemont F, Tadrous M, Harrison M, Law M. How Did a Mandatory Switching Policy for Biosimilars in Canada Impact Uptake and Spending? [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/how-did-a-mandatory-switching-policy-for-biosimilars-in-canada-impact-uptake-and-spending/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/how-did-a-mandatory-switching-policy-for-biosimilars-in-canada-impact-uptake-and-spending/